
Nitrate is a polyatomic ion with the chemical formula NO−
3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

Nitrification is the biological oxidation of ammonia to nitrate via the intermediary nitrite. Nitrification is an important step in the nitrogen cycle in soil. The process of complete nitrification may occur through separate organisms or entirely within one organism, as in comammox bacteria. The transformation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an aerobic process performed by small groups of autotrophic bacteria and archaea.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.

Denitrification is a microbially facilitated process where nitrate (NO3−) is reduced and ultimately produces molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. Facultative anaerobic bacteria perform denitrification as a type of respiration that reduces oxidized forms of nitrogen in response to the oxidation of an electron donor such as organic matter. The preferred nitrogen electron acceptors in order of most to least thermodynamically favorable include nitrate (NO3−), nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O) finally resulting in the production of dinitrogen (N2) completing the nitrogen cycle. Denitrifying microbes require a very low oxygen concentration of less than 10%, as well as organic C for energy. Since denitrification can remove NO3−, reducing its leaching to groundwater, it can be strategically used to treat sewage or animal residues of high nitrogen content. Denitrification can leak N2O, which is an ozone-depleting substance and a greenhouse gas that can have a considerable influence on global warming.

Anammox, an abbreviation for "anaerobic ammonium oxidation", is a globally important microbial process of the nitrogen cycle that takes place in many natural environments. The bacteria mediating this process were identified in 1999, and were a great surprise for the scientific community. In the anammox reaction, nitrite and ammonium ions are converted directly into diatomic nitrogen and water.
Nitrosomonas europaea is a Gram-negative obligate chemolithoautotroph that can derive all its energy and reductant for growth from the oxidation of ammonia to nitrite and lives in several places such as soil, sewage, freshwater, the walls of buildings and on the surface of monuments especially in polluted areas where the air contains high levels of nitrogen compounds.

Nitrosomonas is a genus of Gram-negative bacteria, belonging to the Betaproteobacteria. It is one of the five genera of ammonia-oxidizing bacteria and, as an obligate chemolithoautotroph, uses ammonia as an energy source and carbon dioxide as a carbon source in the presence of oxygen. Nitrosomonas are important in the global biogeochemical nitrogen cycle, since they increase the bioavailability of nitrogen to plants and in the denitrification, which is important for the release of nitrous oxide, a powerful greenhouse gas. This microbe is photophobic, and usually generate a biofilm matrix, or form clumps with other microbes, to avoid light. Nitrosomonas can be divided into six lineages: the first one includes the species Nitrosomonas europea, Nitrosomonas eutropha, Nitrosomonas halophila, and Nitrosomonas mobilis. The second lineage presents the species Nitrosomonas communis, N. sp. I and N. sp. II. The third lineage includes only Nitrosomonas nitrosa. The fourth lineage includes the species Nitrosomonas ureae and Nitrosomonas oligotropha. The fifth and sixth lineages include the species Nitrosomonas marina, N. sp. III, Nitrosomonas estuarii, and Nitrosomonas cryotolerans.
Denitrifying bacteria are a diverse group of bacteria that encompass many different phyla. This group of bacteria, together with denitrifying fungi and archaea, is capable of performing denitrification as part of the nitrogen cycle. Denitrification is performed by a variety of denitrifying bacteria that are widely distributed in soils and sediments and that use oxidized nitrogen compounds such as nitrate and nitrite in the absence of oxygen as a terminal electron acceptor. They metabolize nitrogenous compounds using various enzymes, including nitrate reductase (NAR), nitrite reductase (NIR), nitric oxide reductase (NOR) and nitrous oxide reductase (NOS), turning nitrogen oxides back to nitrogen gas or nitrous oxide.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
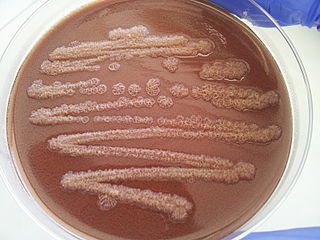
Pseudomonas stutzeri is a Gram-negative soil bacterium that is motile, has a single polar flagellum, and is classified as bacillus, or rod-shaped. While this bacterium was first isolated from human spinal fluid, it has since been found in many different environments due to its various characteristics and metabolic capabilities. P. stutzeri is an opportunistic pathogen in clinical settings, although infections are rare. Based on 16S rRNA analysis, this bacterium has been placed in the P. stutzeri group, to which it lends its name.

In enzymology, a nitrous oxide reductase also known as nitrogen:acceptor oxidoreductase (N2O-forming) is an enzyme that catalyzes the final step in bacterial denitrification, the reduction of nitrous oxide to dinitrogen.
Aerobic denitrification, or co-respiration, the simultaneous use of both oxygen (O2) and nitrate (NO−3) as oxidizing agents, performed by various genera of microorganisms. This process differs from anaerobic denitrification not only in its insensitivity to the presence of oxygen, but also in its higher potential to form nitrous oxide (N2O) as a byproduct.
CandidatusScalindua wagneri is a Gram-negative coccoid-shaped bacterium that was first isolated from a wastewater treatment plant. This bacterium is an obligate anaerobic chemolithotroph that undergoes anaerobic ammonium oxidation (anammox). It can be used in the wastewater treatment industry in nitrogen reactors to remove nitrogenous wastes from wastewater without contributing to fixed nitrogen loss and greenhouse gas emission.

Urine patches in cattle pastures generate large concentrations of the greenhouse gas nitrous oxide through nitrification and denitrification processes in urine-contaminated soils. Over the past few decades, the cattle population has increased more rapidly than the human population. Between the years 2000 and 2050, the cattle population is expected to increase from 1.5 billion to 2.6 billion. When large populations of cattle are packed into pastures, excessive amounts of urine soak into soils. This increases the rate at which nitrification and denitrification occur and produce nitrous oxide. Currently, nitrous oxide is one of the single most important ozone-depleting emissions and is expected to remain the largest throughout the 21st century.
Dissimilatory nitrate reduction to ammonium (DNRA), also known as nitrate/nitrite ammonification, is the result of anaerobic respiration by chemoorganoheterotrophic microbes using nitrate (NO3−) as an electron acceptor for respiration. In anaerobic conditions microbes which undertake DNRA oxidise organic matter and use nitrate (rather than oxygen) as an electron acceptor, reducing it to nitrite, and then to ammonium (NO3− → NO2− → NH4+).

Microbial oxidation of sulfur is the oxidation of sulfur by microorganisms to build their structural components. The oxidation of inorganic compounds is the strategy primarily used by chemolithotrophic microorganisms to obtain energy to survive, grow and reproduce. Some inorganic forms of reduced sulfur, mainly sulfide (H2S/HS−) and elemental sulfur (S0), can be oxidized by chemolithotrophic sulfur-oxidizing prokaryotes, usually coupled to the reduction of oxygen (O2) or nitrate (NO3−). Anaerobic sulfur oxidizers include photolithoautotrophs that obtain their energy from sunlight, hydrogen from sulfide, and carbon from carbon dioxide (CO2).
An oxygen minimum zone (OMZ) is characterized as an oxygen-deficient layer in the world's oceans. Typically found between 200 m to 1500 m deep below regions of high productivity, such as the western coasts of continents. OMZs can be seasonal following the spring-summer upwelling season. Upwelling of nutrient-rich water leads to high productivity and labile organic matter, that is respired by heterotrophs as it sinks down the water column. High respiration rates deplete the oxygen in the water column to concentrations of 2 mg/L or less forming the OMZ. OMZs are expanding, with increasing ocean deoxygenation. Under these oxygen-starved conditions, energy is diverted from higher trophic levels to microbial communities that have evolved to use other biogeochemical species instead of oxygen, these species include nitrate, nitrite, sulphate etc. Several Bacteria and Archea have adapted to live in these environments by using these alternate chemical species and thrive. The most abundant phyla in OMZs are Pseudomonadota, Bacteroidota, Actinomycetota, and Planctomycetota.

NC10 is a bacterial phylum with candidate status, meaning its members remain uncultured to date. The difficulty in producing lab cultures may be linked to low growth rates and other limiting growth factors.

Candidatus "Methylomirabilis oxyfera" is a candidate species of Gram-negative bacteria belonging to the NC10 phylum, characterized for its capacity to couple anaerobic methane oxidation with nitrite reduction in anoxic environments. To acquire oxygen for methane oxidation, M. oxyfera utilizes an intra-aerobic pathway through the reduction of nitrite (NO2) to dinitrogen (N2) and oxygen.










