In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

The avermectins are a series of drugs and pesticides used to treat parasitic worms and insect pests. They are a group of 16-membered macrocyclic lactone derivatives with potent anthelmintic and insecticidal properties. These naturally occurring compounds are generated as fermentation products by Streptomyces avermitilis, a soil actinomycete. Eight different avermectins were isolated in four pairs of homologue compounds, with a major (a-component) and minor (b-component) component usually in ratios of 80:20 to 90:10. Avermectin B1, a mixture of B1a and B1b, is the drug and pesticide abamectin. Other anthelmintics derived from the avermectins include ivermectin, selamectin, doramectin, eprinomectin.

Ascofuranone is an antibiotic produced by various ascomycete fungi including Acremonium sclerotigenum that inhibits the Trypanosoma brucei alternative oxidase and is a lead compound in efforts to produce other drugs targeting this enzyme for the treatment of sleeping sickness. The compound is effective both in vitro cell culture and in infections in mice.
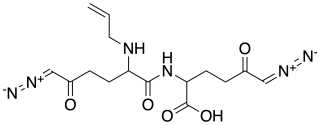
Alazopeptin is an antibiotic, with moderate anti-trypanosomal and antitumor activity. It was originally isolated from Streptacidiphilus griseoplanus, sourced from soil near Williamsburg, Iowa. It is also isolated from Kitasatospora azatica It is still largely produced via fermentation broths of that organism. Structurally, alazopeptin is a tripeptide and contains 2 molecules of 6-diazo-5-oxo-L-norleucine and one molecule of L-alanine. In 2021 the biosynthetic pathway of alazopeptin was elucidated.
Aspergillus sydowii is a pathogenic fungus that causes several diseases in humans. It has been implicated in the death of sea fan corals in the Caribbean Sea.

Neoxaline is a bio-active Aspergillus japonicus isolate. It is an antimitotic agent and shows weak inhibitory activity of blood platelet aggregation. It weakly stimulates the central nervous system. It has been synthesized through the "highly stereoselective introduction of a reverse prenyl group to create a quaternary carbon stereocenter using (−)-3a-hydroxyfuroindoline as a building block, construction of the indoline spiroaminal via cautious stepwise oxidations with cyclizations from the indoline, assembly of (Z)-dehydrohistidine, and photoisomerization of unnatural (Z)-neoxaline to the natural (E)-neoxaline."

Duclauxin is a chemical compound isolated from Penicillium duclauxi.
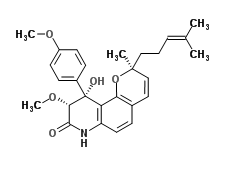
Yaequinolone J1 is an antibiotic made by Penicillium.

Citreorosein is a polyketide made by Penicillium that has antimicrobial activity.

Aspergillusenes are a group of chemical compounds first isolated from a strain of sea fan-derived fungus Aspergillus sydowii. They are sesquiterpenes of the bisabolane-type.
Penicillium chermesinum is an anamorph fungus species of the genus of Penicillium which was isolated from soil from Nova Scotia in Canada.Penicillium chermesinum produces plastatin, luteosporin, xanthomegnin, azaphilones, p-terphenyls and costaclavine.
Penicillium corylophilum is a species of the genus of Penicillium which occurs in damp buildings in United States, Canada and western Europe but it can also be found in a variety of foods and mosquitoes. Penicillium corylophilum produces the alkaloid epoxyagroclavine and citrinin and is a pathogen to mosquitoes.
Penicillium paxilli is an anamorph, saprophytic species of the genus Penicillium which produces paxilline, paxisterol, penicillone, pyrenocine A, paspaline B and verruculogene. Penicillium paxilli is used as a model to study the biochemistry of the indol-diterepene biosynthesis
Penicillium pinophilum is a species of fungus in the genus Penicillium which was isolated from a radio set in Papua New Guinea. Penicillium pinophilum produces 3-O-methylfunicone and mycophenolic acid
Penicillium turbatum is an anamorph species of fungus in the genus Penicillium which was isolated from Taxus baccata. Penicillium turbatum produces pipolythiopiperazinedione-antibiotics, hyalodendrin A and hadacitin.
Penicillium viticola is a species of fungus in the genus Penicillium which was isolated from grapes in Yamanashi Prefecture in Japan. Penicillium viticola produces calcium malate
Streptomyces pseudovenezuelae is a bacterium species from the genus of Streptomyces which has been isolated from lead polluted soil in China. Streptomyces pseudovenezuelae produces chloramphenicol and setomimycin.
Streptomyces roseofulvus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces roseofulvus produces deoxyfrenolicin and frenolicin B.

The phomoxanthones are a loosely defined class of natural products. The two founding members of this class are phomoxanthone A and phomoxanthone B. Other compounds were later also classified as phomoxanthones, although a unifying nomenclature has not yet been established. The structure of all phomoxanthones is derived from a dimer of two covalently linked tetrahydroxanthones, and they differ mainly in the position of this link as well as in the acetylation status of their hydroxy groups. The phomoxanthones are structurally closely related to other tetrahydroxanthone dimers such as the secalonic acids and the eumitrins. While most phomoxanthones were discovered in fungi of the genus Phomopsis, most notably in the species Phomopsis longicolla, some have also been found in Penicillium sp.
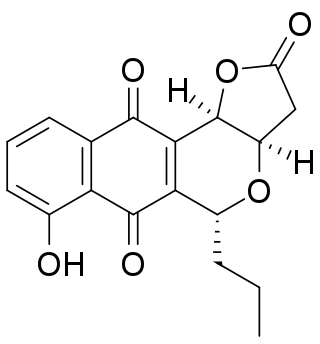
Frenolicin B is an antibiotic and antitumor agent with the molecular formula C18H16O6 which is produced by the bacterium Streptomyces roseofulvus. Frenolicin B is a selective inhibitor of glutaredoxin 3 and peroxiredoxin 1.









