
The Cape Fear River is a 191.08-mile-long blackwater river in east-central North Carolina. It flows into the Atlantic Ocean near Cape Fear, from which it takes its name. The river is formed at the confluence of the Haw River and the Deep River in the town of Moncure, North Carolina. Its river basin is the largest in the state: 9,149 sq mi.
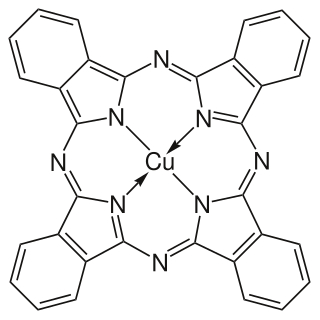
Copper phthalocyanine (CuPc), also called phthalocyanine blue, phthalo blue and many other names, is a bright, crystalline, synthetic blue pigment from the group of dyes based on phthalocyanines. Its brilliant blue is frequently used in paints and dyes. It is highly valued for its superior properties such as light fastness, tinting strength, covering power and resistance to the effects of alkalis and acids. It has the appearance of a blue powder, insoluble in most solvents including water.

Perfluorooctanesulfonic acid (PFOS) is a chemical compound having an eight-carbon fluorocarbon chain and a sulfonic acid functional group and thus a perfluorosulfonic acid. It is an anthropogenic (man-made) fluorosurfactant, now regarded as a global pollutant. PFOS was the key ingredient in Scotchgard, a fabric protector made by 3M, and related stain repellents. The acronym "PFOS" refers to the parent sulfonic acid and to various salts of perfluorooctanesulfonate. These are all colorless or white, water-soluble solids. Although of low acute toxicity, PFOS has attracted much attention for its pervasiveness and environmental impact. It was added to Annex B of the Stockholm Convention on Persistent Organic Pollutants in May 2009.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is an organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that is a colorless solid at room temperature and is soluble in water and a wide variety of organic substances.
Perfluorononanoic acid, or PFNA, is a synthetic perfluorinated carboxylic acid and fluorosurfactant that is also an environmental contaminant found in people and wildlife along with PFOS and PFOA.
Per- and polyfluoroalkyl substances are a group of synthetic organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain; there are 7 million such chemicals according to PubChem. PFAS came into use after the invention of Teflon in 1938 to make fluoropolymer coatings and products that resist heat, oil, stains, grease, and water. They are now used in products including waterproof fabric such as Nylon, yoga pants, carpets, shampoo, feminine hygiene products, mobile phone screens, wall paint, furniture, adhesives, food packaging, heat-resistant non-stick cooking surfaces such as Teflon, firefighting foam, and the insulation of electrical wire. PFAS are also used by the cosmetic industry in most cosmetics and personal care products, including lipstick, eye liner, mascara, foundation, concealer, lip balm, blush, and nail polish.

Perfluorobutanesulfonic acid (PFBS) is a PFAS chemical compound having a four-carbon fluorocarbon chain and a sulfonic acid functional group. It is stable and unreactive because of the strength of carbon–fluorine bonds. It can occur in the form of a colorless liquid or a corrosive solid. Its conjugate base is perfluorobutanesulfonate which functions as the hydrophobe in fluorosurfactants.
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound that lacks C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common functional groups in PFCs are OH, CO2H, chlorine, O, and SO3H. Electrofluorination is the predominant method for PFC production. Due to their chemical stability, some of these perfluorinated compounds bioaccumulate.
Perfluoroalkyl carboxylic acids (PFCAs), or perfluorocarboxylic acids are compounds of the formula CnF(2n+1)CO2H that belong to the class of per- and polyfluoroalkyl substances. The simplest example is trifluoroacetic acid. These compounds are organofluorine analogues of ordinary carboxylic acids, but they are stronger by several pKa units and they exhibit great hydrophobic character. Perfluoroalkyl dicarboxylic acids (PFdiCAs) are also known, e.g. C2F4(CO2H)2.

Perfluorobutanoic acid (PFBA) is a perfluoroalkyl carboxylic acid with the formula C3F7CO2H. As the perfluorinated derivative of butyric acid, this colourless liquid is prepared by electrofluorination of the corresponding butyryl fluoride.
Sodium salts are salts composed of a sodium cation and the conjugate base anion of some inorganic or organic acids. They can be formed by the neutralization of such acids with sodium hydroxide.
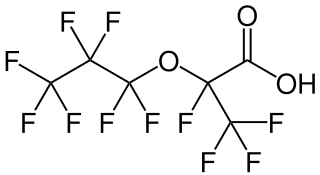
FRD-903 is a chemical compound that is among the class of per- and polyfluoroalkyl substances (PFASs). More specifically, this synthetic petrochemical is also described as a perfluoroalkyl ether carboxylic acid (PFECA) and a Fluorointermediate. It is not biodegradable and is not hydrolyzed by water.
GenX is a Chemours trademark name for a synthetic, short-chain organofluorine chemical compound, the ammonium salt of hexafluoropropylene oxide dimer acid (HFPO-DA). It can also be used more informally to refer to the group of related fluorochemicals that are used to produce GenX. DuPont began the commercial development of GenX in 2009 as a replacement for perfluorooctanoic acid.

Perfluorosulfonic acids (PFSAs) are chemical compounds of the formula CnF(2n+1)SO3H and thus belong to the family of perfluorinated and polyfluorinated alkyl compounds (PFASs). The simplest example of a perfluorosulfonic acid is the trifluoromethanesulfonic acid. Perfluorosulfonic acids with six or more perfluorinated carbon atoms, i.e. from perfluorohexanesulfonic acid onwards, are referred to as long-chain.
Perfluorohexanesulfonic acid (PFHxS) is a synthetic chemical compound. It is one of many compounds collectively known as per- and polyfluoroalkyl substances (PFASs). It is an anionic fluorosurfactant and a persistent organic pollutant with bioaccumulative properties. Although the use of products containing PFHxS and other PFASs have been banned or are being phased out in many jurisdictions, it remains ubiquitous in many environments and within the general population, and is one of the most commonly detected PFASs.
Sulfluramid (N-EtFOSA) is a chemical compound from the group of sulfonic acid amides and per- and polyfluoroalkyl substances (PFASs) that is effective as an insecticide.
Remediation of per- and polyfluoroalkyl substances refers to the destruction or removal of perfluoroalkyl substances (PFASs) from the environment. PFAS's are a group of synthetic organofluorine compounds that are used to produce diverse products such as non-stick cookware and firs-fighting foams. Known as "everywhere chemicals", they have attracted great concern as chronic poisons. Because they are pervasive and have adverse effects, much interest has focused on their removal.

Perfluoro(4-ethylcyclohexane)sulfonic acid (PFEtCHxS) is a synthetic perfluorinated alkyl substance that has been used as a surfactant and in various industrial applications.

The TOP Assay is a laboratory method developed in 2012 that oxidatively converts (unknown) precursor compounds of perfluorocarboxylic acids (PFCAs) into the latter. This makes quantification possible. Potassium peroxodisulfate is used. This sum parameter can be used to determine the concentration of precursor compounds present by comparing the sample before and after the application of the TOP Assay.


 [1]
[1] 











