
A resin is a solid or highly viscous liquid that can be converted into a polymer. Resins may be biological or synthetic in origin, but are typically harvested from plants. Resins are mixtures of organic compounds, and predominantly terpenes. Well known resins include amber, hashish, frankincense, myrrh and the animal-derived resin, shellac. Resins are commonly used in varnishes, adhesives, food additives, incenses and perfumes.

Turpentine is a fluid obtained by the distillation of resin harvested from living trees, mainly pines. Principally used as a specialized solvent, it is also a source of material for organic syntheses.

Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. In plants, terpenes and terpenoids are important mediators of ecological interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control.
An antimicrobial is an agent that kills microorganisms (microbicide) or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals are used against fungi. They can also be classified according to their function. Antimicrobial medicines to treat infection are known as ⠀⠀antimicrobial chemotherapy, while antimicrobial drugs are used to prevent infection, which known as antimicrobial prophylaxis.

Pinus roxburghii, commonly known as chir pine or longleaf Indian pine, is a species of pine tree native to the Himalayas. It was named after William Roxburgh.

In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure [NR4]+, where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.

Linalool refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants. Together with geraniol, nerol, citronellol, linalool is one of the rose alcohols. Linalool has multiple commercial applications, the majority of which are based on its pleasant scent.

Limonene is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the essential oil of citrus fruit peels. The (+)-isomer, occurring more commonly in nature as the fragrance of oranges, is a flavoring agent in food manufacturing. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The less common (-)-isomer has a piny, turpentine-like odor, and is found in the edible parts of such plants as caraway, dill, and bergamot orange plants.

The oligodynamic effect is a biocidal effect of metals, especially heavy metals, that occurs even in low concentrations. This effect is attributed to the antibacterial behavior of metal ions, which are absorbed by bacteria upon contact and damage their cell membranes.
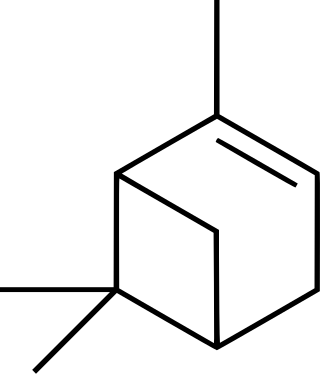
Pinene is a collection of unsaturated bicyclic monoterpenes. Two geometric isomers of pinene are found in nature, α-pinene and β-pinene. Both are chiral. As the name suggests, pinenes are found in pines. Specifically, pinene is the major component of the liquid extracts of conifers. Pinenes are also found in many non-coniferous plants such as camphorweed (Heterotheca) and big sagebrush.
Naval stores refers to the industry that produces rosin, turpentine, tall oil, pine oil, and other oleoresin collected from conifers. The term was originally applied to the compounds used in building and maintaining wooden sailing ships. Presently, pine compounds produced by the naval stores industry are used to manufacture soap, paint, varnish, shoe polish, lubricants, linoleum, and roofing materials.
Longifolene is a common sesquiterpene. It is an oily liquid hydrocarbon found primarily in the high-boiling fraction of certain pine resins. The name is derived from that of a pine species from which the compound was isolated. It is a tricyclic chiral molecule. The enantiomer commonly found in pines and other higher plants exhibits a positive optical rotation of +42.73°. The other enantiomer is found in small amounts in certain fungi and liverworts.

3-Carene is a bicyclic monoterpene consisting of fused cyclohexene and cyclopropane rings. It occurs as a constituent of turpentine, with a content as high as 42% depending on the source. Carene has a sweet and pungent odor, best described as a combination of fir needles, musky earth, and damp woodlands.
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As with other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango. It is produced industrially by isomerization of the more common alpha-pinene using a solid acid catalyst such as titanium dioxide.
Peracetic acid (also known as peroxyacetic acid, or PAA) is an organic compound with the formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive.

The naval stores industry produces and markets products derived from the oleoresin of pine trees, including rosin, tall oil, pine oil, and turpentine. It does this by collecting and processing organic forest products refined from slash pine and longleaf pine trees. The naval stores industry was associated with the maintenance of the wooden ships and sailing tackle of pre-20th century navies, which were caulked and waterproofed using the pitch of the pine tree.
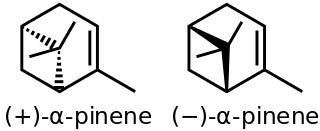
α-Pinene is an organic compound of the terpene class. It is one of the two isomers of pinene, the other being β-pinene. An alkene, it contains a reactive four-membered ring. It is found in the oils of many species of coniferous trees, notably Pinus and Picea species. It is also found in the essential oil of rosemary and Satureja myrtifolia. Both enantiomers are known in nature; (1S,5S)- or (−)-α-pinene is more common in European pines, whereas the (1R,5R)- or (+)-α-isomer is more common in North America. The enantiomers' racemic mixture is present in some oils such as eucalyptus oil and orange peel oil.

β-Pinene is a monoterpene, an organic compound found in plants. It is the less abundant of the two isomers of pinene, the other being α-pinene. It is a colorless liquid soluble in alcohol, but not water. It has a woody-green pine-like smell.
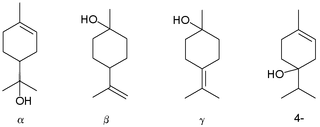
Terpineol is any of four isomeric monoterpenoids. Terpenoids are terpene that are modified by the addition of a functional group, in this case, an alcohol. Terpineols have been isolated from a variety of sources such as cardamom, cajuput oil, pine oil, and petitgrain oil. Four isomers exist: α-terpineol, β-terpineol, γ-terpineol, and terpinen-4-ol. β-Terpineol and γ-terpineol differ only by the location of the double bond. Terpineol is usually a mixture of these isomers with α-terpineol as the major constituent.
In chemistry, a fatty amine is loosely defined as any amine possessing a mostly linear hydrocarbon chain of eight or more carbon atoms. They are typically prepared from the more abundant fatty acids, with vegetable or seed-oils being the ultimate starting material. As such they are often mixtures of chain lengths, ranging up to about C22. They can be classified as oleochemicals. Commercially important members include coco amine, oleylamine, tallow amine, and soya amine. These compounds and their derivatives are used as fabric softeners, froth flotation agents, corrosion inhibitors, lubricants and friction modifiers. They are also the basis for a variety of cosmetic formulations.
















