
Poliovirus, the causative agent of polio, is a serotype of the species Enterovirus C, in the family of Picornaviridae. There are three poliovirus serotypes: types 1, 2, and 3.

Defective interfering particles (DIPs), also known as defective interfering viruses, are spontaneously generated virus mutants in which a critical portion of the particle's genome has been lost due to defective replication or non-homologous recombination. The mechanism of their formation is presumed to be as a result of template-switching during replication of the viral genome, although non-replicative mechanisms involving direct ligation of genomic RNA fragments have also been proposed. DIPs are derived from and associated with their parent virus, and particles are classed as DIPs if they are rendered non-infectious due to at least one essential gene of the virus being lost or severely damaged as a result of the defection. A DIP can usually still penetrate host cells, but requires another fully functional virus particle to co-infect a cell with it, in order to provide the lost factors.
An oncovirus or oncogenic virus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.
Circoviridae is a family of DNA viruses. Birds and mammals serve as natural hosts. There are 101 species in this family, assigned to 2 genera. Diseases associated with this family include: PCV-2: postweaning multisystemic wasting syndrome; CAV: chicken infectious anemia.

Barnaviridae is a family of non-enveloped, positive-strand RNA viruses. Cultivated mushrooms serve as natural hosts. The family has one genus, Barnavirus, which contains one species: Mushroom bacilliform virus. Diseases associated with this family includes La France disease.
Porcine circoviral disease (PCVD), also known as porcine circovirus associated disease (PCVAD), is a disease seen in domestic pigs. This disease causes illness in piglets, with clinical signs including progressive loss of body condition, visibly enlarged lymph nodes, difficulty in breathing, and sometimes diarrhea, pale skin, and jaundice. PCVD is very damaging to the pig-producing industry and has been reported worldwide. PCVD is caused by Porcine circovirus 2 (PCV-2).
Betaarterivirus suid 1, commonly Porcine reproductive and respiratory syndrome virus (PRRSV), is a virus that causes a disease of pigs, called porcine reproductive and respiratory syndrome (PRRS), also known as blue-ear pig disease. This economically important, panzootic disease causes reproductive failure in breeding stock and respiratory tract illness in young pigs.
HHV Latency Associated Transcript is a length of RNA which accumulates in cells hosting long-term, or latent, Human Herpes Virus (HHV) infections. The LAT RNA is produced by genetic transcription from a certain region of the viral DNA. LAT regulates the viral genome and interferes with the normal activities of the infected host cell.
Pestivirus is a genus of viruses, in the family Flaviviridae. Viruses in the genus Pestivirus infect mammals, including members of the family Bovidae and the family Suidae. There are 11 species in this genus. Diseases associated with this genus include: hemorrhagic syndromes, abortion, and fatal mucosal disease.
Porcine parvovirus (PPV), a virus in the species Ungulate protoparvovirus 1 of genus Protoparvovirus in the virus family Parvoviridae, causes reproductive failure of swine characterized by embryonic and fetal infection and death, usually in the absence of outward maternal clinical signs. The disease develops mainly when seronegative dams are exposed oronasally to the virus anytime during about the first half of gestation, and conceptuses are subsequently infected transplacentally before they become immunocompetent. There is no definitive evidence that infection of swine other than during gestation is of any clinical or economic significance. The virus is ubiquitous among swine throughout the world and is enzootic in most herds that have been tested. Diagnostic surveys have indicated that PPV is the major infectious cause of embryonic and fetal death. In addition to its direct causal role in reproductive failure, PPV can potentiate the effects of porcine circovirus type II (PCV2) infection in the clinical course of postweaning multisystemic wasting syndrome (PMWS).

Herpes simplex virus1 and 2, also known by their taxonomic names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are very common and contagious. They can be spread when an infected person begins shedding the virus.

Psittacine beak and feather disease (PBFD) is a viral disease affecting all Old World and New World parrots. The causative virus—beak and feather disease virus (BFDV)—belongs to the taxonomic genus Circovirus, family Circoviridae. It attacks the feather follicles and the beak and claw matrices of the bird, causing progressive feather, claw and beak malformation and necrosis. In later stages of the disease, feather shaft constriction occurs, hampering development until eventually all feather growth stops. It occurs in an acutely fatal form and a chronic form.
Anelloviridae is a family of viruses. They are classified as vertebrate viruses and have a non-enveloped capsid, which is round with isometric, icosahedral symmetry and has a triangulation number of 3.

Sobemovirus is a genus of non-enveloped, positive-strand RNA viruses which infect plants.. Plants serve as natural hosts. There are 21 species in this genus. Diseases associated with this genus include: mosaics and mottles.
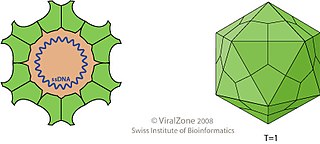
Circovirus is a genus of viruses, in the family Circoviridae. Birds and pigs serve as natural hosts, though dogs have been shown to be infected as well. It is a single stranded DNA virus (ssDNA). There are 49 species in this genus. Some members of this genus cause disease: PCV-1 is non pathogenic, while PCV-2 causes postweaning multisystemic wasting syndrome (PMWS).
This glossary of virology is a list of definitions of terms and concepts used in virology, the study of viruses, particularly in the description of viruses and their actions. Related fields include microbiology, molecular biology, and genetics.
Canine circovirus, first isolated in 2012, is a small non-enveloped, icosahedral, single-stranded DNA virus that infects domestic dogs and wild canids exclusively. It is a member of the Circoviridae family and the genus Circovirus. There are currently 11 species of known circoviruses that have been identified to affect a wide variety of birds and mammals. As seen with all extensively studied circoviruses, the diameter ranges between approximately 15 and 25 nanometers. The icosahedral triangulation number is 1, the smallest size a viral capsid can be, in which there are a total of 60 protein subunits that make up the capsid. CaCV is not to be confused with canine coronavirus, another diarrhea-causing agent within the family Coronaviridae, or porcine circoviruses which are a members of the same genus as CaCV but only seen in pigs. CaCV was the first Circovirus to be identified that infects a mammal species other than pigs.
Torque teno sus virus, belonging to the family Anelloviridae, is a group of virus strains that are non-enveloped, with a single-stranded circular DNA genome ranging from 2.6 to 2.8 kb in size. These swine infecting anelloviruses are divided into two genera: Iotatorquevirus and Kappatorquevirus. Torque teno sus virus has been found in pigs worldwide. TTSuVs are mainly transmitted by fecal-oral route. The prevalence of these viruses is relatively high. For now, there is not known disease caused exclusively by TTSuV. There is the possibility that TTSuV may worsen the progression of other diseases and therefore increase the economic losses for pig industry.

Redondoviruses are a family of human-associated DNA viruses. Their name derives from the inferred circular structure of the viral genome . Redondoviruses have been identified in DNA sequence based surveys of samples from humans, primarily samples from the oral cavity and upper airway.

Monodnaviria is a realm of viruses that includes all single-stranded DNA viruses that encode an endonuclease of the HUH superfamily that initiates rolling circle replication of the circular viral genome. Viruses descended from such viruses are also included in the realm, including certain linear single-stranded DNA (ssDNA) viruses and circular double-stranded DNA (dsDNA) viruses. These atypical members typically replicate through means other than rolling circle replication.













