
Transglutaminases are enzymes that in nature primarily catalyze the formation of an isopeptide bond between γ-carboxamide groups ( -(C=O)NH2 ) of glutamine residue side chains and the ε-amino groups ( -NH2 ) of lysine residue side chains with subsequent release of ammonia ( NH3 ). Lysine and glutamine residues must be bound to a peptide or a protein so that this cross-linking (between separate molecules) or intramolecular (within the same molecule) reaction can happen. Bonds formed by transglutaminase exhibit high resistance to proteolytic degradation (proteolysis). The reaction is

Glutamate dehydrogenase is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typically, the α-ketoglutarate to glutamate reaction does not occur in mammals, as glutamate dehydrogenase equilibrium favours the production of ammonia and α-ketoglutarate. Glutamate dehydrogenase also has a very low affinity for ammonia, and therefore toxic levels of ammonia would have to be present in the body for the reverse reaction to proceed. However, in brain, the NAD+/NADH ratio in brain mitochondria encourages oxidative deamination. In bacteria, the ammonia is assimilated to amino acids via glutamate and aminotransferases. In plants, the enzyme can work in either direction depending on environment and stress. Transgenic plants expressing microbial GLDHs are improved in tolerance to herbicide, water deficit, and pathogen infections. They are more nutritionally valuable.

An isopeptide bond is a type of amide bond formed between a carboxyl group of one amino acid and an amino group of another. An isopeptide bond is the linkage between the side chain amino or carboxyl group of one amino acid to the α-carboxyl, α-amino group, or the side chain of another amino acid. In a typical peptide bond, also known as eupeptide bond, the amide bond always forms between the α-carboxyl group of one amino acid and the α-amino group of the second amino acid. Isopeptide bonds are rarer than regular peptide bonds. Isopeptide bonds lead to branching in the primary sequence of a protein. Proteins formed from normal peptide bonds typically have a linear primary sequence.

Glutaminase is an amidohydrolase enzyme that generates glutamate from glutamine. Glutaminase has tissue-specific isoenzymes. Glutaminase has an important role in glial cells.
Carbamoyl phosphate synthetase (glutamine-hydrolysing) is an enzyme that catalyzes the reactions that produce carbamoyl phosphate in the cytosol. Its systemic name is hydrogen-carbonate:L-glutamine amido-ligase .

In enzymology, a NAD+ synthase (glutamine-hydrolysing) (EC 6.3.5.1) is an enzyme that catalyzes the chemical reaction
In enzymology, an adenylyl-[glutamate---ammonia ligase] hydrolase (EC 3.1.4.15) is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-methyleneglutaminase (EC 3.5.1.67) is an enzyme that catalyzes the chemical reaction
In enzymology, a D-glutaminase (EC 3.5.1.35) is an enzyme that catalyzes the chemical reaction
In enzymology, a formimidoylglutamate deiminase (EC 3.5.3.13) is an enzyme that catalyzes the chemical reaction
In enzymology, a glutamin-(asparagin-)ase (EC 3.5.1.38) is an enzyme that catalyzes the chemical reaction

In enzymology, an omega-amidase (EC 3.5.1.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a peptidyl-glutaminase (EC 3.5.1.43) is an enzyme that catalyzes the chemical reaction

In enzymology, a protein-arginine deiminase (EC 3.5.3.15) is an enzyme that catalyzes a form of post translational modification called arginine de-imination or citrullination:
In enzymology, a theanine hydrolase (EC 3.5.1.65) is an enzyme that catalyzes the chemical reaction
In enzymology, a D-glutamyltransferase (EC 2.3.2.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a glutaminyl-peptide cyclotransferase (EC 2.3.2.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a glutamine-pyruvate transaminase is an enzyme that catalyzes the chemical reaction
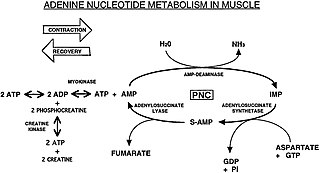
The Purine Nucleotide Cycle is a metabolic pathway in protein metabolism requiring the amino acids aspartate and glutamate. The cycle is used to regulate the levels of adenine nucleotides, in which ammonia and fumarate are generated. AMP coverts into IMP and the byproduct ammonia. IMP converts to S-AMP (adenylosuccinate), which then coverts to AMP and the byproduct fumarate. The fumarate goes on to produce ATP (energy) via oxidative phosphorylation as it enters the Krebs cycle and then the electron transport chain. Lowenstein first described this pathway and outlined its importance in processes including amino acid catabolism and regulation of flux through glycolysis and the Krebs cycle.
Asparagine synthase (glutamine-hydrolysing) (EC 6.3.5.4, asparagine synthetase (glutamine-hydrolysing), glutamine-dependent asparagine synthetase, asparagine synthetase B, AS, AS-B) is an enzyme with systematic name L-aspartate:L-glutamine amido-ligase (AMP-forming). This enzyme catalyses the following chemical reaction









