
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel and an oxidizing agent into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.
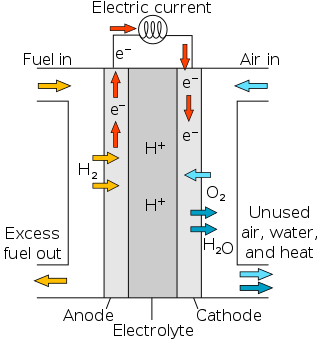
Proton-exchange membrane fuel cells (PEMFC), also known as polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging alkaline fuel-cell technology, which was used in the Space Shuttle.

Direct methanol fuel cells or DMFCs are a subcategory of proton-exchange membrane fuel cells in which methanol is used as the fuel and a special proton-conducting polymer as the membrane (PEM). Their main advantage is low temperature operation and the ease of transport of methanol, an energy-dense yet reasonably stable liquid at all environmental conditions.

The methanol economy is a suggested future economy in which methanol and dimethyl ether replace fossil fuels as a means of energy storage, ground transportation fuel, and raw material for synthetic hydrocarbons and their products. It offers an alternative to the proposed hydrogen economy or ethanol economy, although these concepts are not exclusive. Methanol can be produced from a variety of sources including fossil fuels as well as agricultural products and municipal waste, wood and varied biomass. It can also be made from chemical recycling of carbon dioxide.
Micro combined heat and power, micro-CHP, μCHP or mCHP is an extension of the idea of cogeneration to the single/multi family home or small office building in the range of up to 50 kW. Usual technologies for the production of heat and power in one common process are e.g. internal combustion engines, micro gas turbines, stirling engines or fuel cells.
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen gas. This is their essential function when incorporated into a membrane electrode assembly (MEA) of a proton-exchange membrane fuel cell or of a proton-exchange membrane electrolyser: separation of reactants and transport of protons while blocking a direct electronic pathway through the membrane.
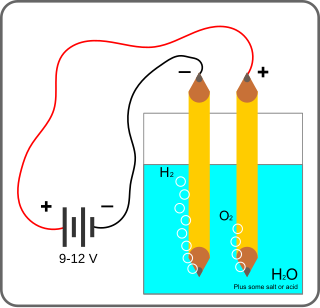
Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C.
Formic acid fuel cells (direct formic acid fuel cells or DFAFCs) are a subcategory of direct liquid-feed fuel cells (DLFCs), in which the liquid fuel is directly oxidized (electrochemically) at the anode instead of reforming to produce hydrogen. Formic acid-based fuel cells represent a promising energy supply system in terms of high volumetric energy density, theoretical energy efficiency, and theoretical open-circuit voltage. They are also able to overcome certain problems inherent to traditional hydrogen (H2) feed fuel cells such as safe handling, storage, and H2 transportation.
Direct-ethanol fuel cells or DEFCs are a category of fuel cell in which ethanol is fed directly into the cell. They have been used as a model to investigate a range of fuel cell concepts including the use of PEM.
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Most hydrogen is gray hydrogen made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as blue hydrogen.
Hydrogen technologies are technologies that relate to the production and use of hydrogen as a part hydrogen economy. Hydrogen technologies are applicable for many uses.
Hydrogen purification is any technology used to purify hydrogen. The impurities in hydrogen gas depend on the source of the H2, e.g., petroleum, coal, electrolysis, etc. The required purity is determined by the application of the hydrogen gas. For example, ultra-high purified hydrogen is needed for applications like proton exchange membrane fuel cells.

High-pressure electrolysis (HPE) is the electrolysis of water by decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to the passing of an electric current through the water. The difference with a standard proton exchange membrane electrolyzer is the compressed hydrogen output around 12–20 megapascals (120–200 bar) at 70 °C. By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated, the average energy consumption for internal differential pressure compression is around 3%.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
An anion exchange membrane (AEM) is a semipermeable membrane generally made from ionomers and designed to conduct anions but reject gases such as oxygen or hydrogen.
Membraneless Fuel Cells convert stored chemical energy into electrical energy without the use of a conducting membrane as with other types of Fuel Cells. In Laminar Flow Fuel Cells (LFFC) this is achieved by exploiting the phenomenon of non-mixing laminar flows where the interface between the two flows works as a proton/ion conductor. The interface allows for high diffusivity and eliminates the need for costly membranes. The operating principles of these cells mean that they can only be built to millimeter-scale sizes. The lack of a membrane means they are cheaper but the size limits their use to portable applications which require small amounts of power.

An alkaline anion-exchange membrane fuel cell (AAEMFC), also known as anion-exchange membrane fuel cells (AEMFCs), alkaline membrane fuel cells (AMFCs), hydroxide-exchange membrane fuel cells (HEMFCs), or solid alkaline fuel cells (SAFCs) is a type of alkaline fuel cell that uses an anion-exchange membrane to separate the anode and cathode compartments.

Carbon Recycling International (CRI) is an Icelandic limited liability company which has developed a technology designed to produce renewable methanol, also known as e-methanol, from carbon dioxide and hydrogen, using water electrolysis or, alternatively, hydrogen captured from industrial waste gases. The technology is trademarked by CRI as Emissions-to-Liquids (ETL) and the renewable methanol produced by CRI is trademarked as Vulcanol. In 2011 CRI became the first company to produce and sell liquid renewable transport fuel produced using only carbon dioxide, water and electricity from renewable sources.
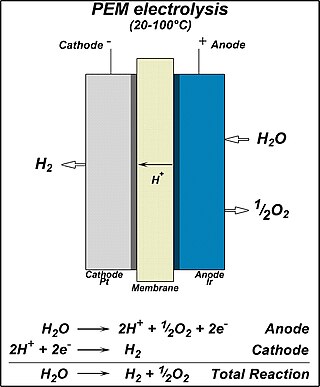
Proton exchange membrane(PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer. It involves a proton-exchange membrane.
High Temperature Proton Exchange Membrane fuel cells (HT-PEMFC), also known as High Temperature Polymer Electrolyte Membrane fuel cells, are a type of PEM fuel cells which can be operated at temperatures between 120 and 200°C. HT-PEM fuel cells are used for both stationary and portable applications. The HT-PEM fuel cell is usually supplied with hydrogen-rich gas like reformate gas formed by reforming of methanol, ethanol, natural gas or LPG.








