External links
| | This spectroscopy-related article is a stub. You can help Wikipedia by expanding it. |
| | This scattering–related article is a stub. You can help Wikipedia by expanding it. |
Saturated spectroscopy is the method by which the exact energy of the hyperfine transitions within an atom can be found. When a monochromatic light is shone through an atom, the absorption cross-section is broadened due to Doppler broadening. Saturated spectroscopy allows the doppler broadened peak to be resolved so that the exact transitions can be found.
More than a decade after the first demonstration of spectral hole burning (or Lamb dip, a result of saturated absorption process) inside HeNe laser cavity at 1.1 μm in 1962, the greater majority of SA spectroscopy research was carried out with gas lasers and molecules in the mid-infrared.
But because SA requires high laser intensity, and the gas molecules usually have widely spread strong absorption spectra only in the mid-IR, while compact widely tunable mid-IR lasers were slow to develop, the SA technique has not been widely used for molecular chemical analysis besides precision metrology, which only been limited to the isolated wavelengths of HeNe and CO2 lasers and limited number of molecules.

Infrared spectroscopy is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers, symbol μm, which are related to the wavenumber in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer. Two-dimensional IR is also possible as discussed below.

Spectroscopy is the general field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO)
The Mössbauer effect, or recoilless nuclear resonance fluorescence, is a physical phenomenon discovered by Rudolf Mössbauer in 1958. It involves the resonant and recoil-free emission and absorption of gamma radiation by atomic nuclei bound in a solid. Its main application is in Mössbauer spectroscopy.

Laser cooling and laser trapping include a number of techniques in which atomic and molecular samples are cooled down to near absolute zero. Laser cooling techniques rely on the fact that when an object absorbs and re-emits a photon its momentum changes. For an ensemble of particles, their thermodynamic temperature is proportional to the variance in their velocity. That is, more homogeneous velocities among particles corresponds to a lower temperature. Laser cooling techniques combine atomic spectroscopy with the aforementioned mechanical effect of light to compress the velocity distribution of an ensemble of particles, thereby cooling the particles. The 1997 Nobel Prize in Physics was awarded to Claude Cohen-Tannoudji, Steven Chu, and William Daniel Phillips "for development of methods to cool and trap atoms with laser light".
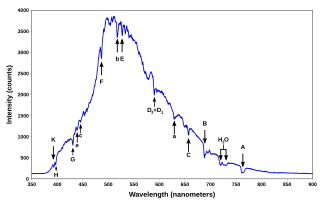
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected ones of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible.

Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum.

A helium–neon laser or He-Ne laser, is a type of gas laser whose high energetic medium gain medium consists of a mixture of 10:1 ratio of helium and neon at a total pressure of about 1 torr inside of a small electrical discharge. The best-known and most widely used He-Ne laser operates at a wavelength of 632.8 nm, in the red part of the visible spectrum.
Tunable diode laser absorption spectroscopy is a technique for measuring the concentration of certain species such as methane, water vapor and many more, in a gaseous mixture using tunable diode lasers and laser absorption spectrometry. The advantage of TDLAS over other techniques for concentration measurement is its ability to achieve very low detection limits. Apart from concentration, it is also possible to determine the temperature, pressure, velocity and mass flux of the gas under observation. TDLAS is by far the most common laser based absorption technique for quantitative assessments of species in gas phase.
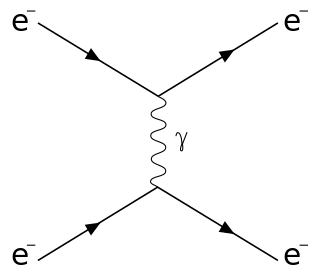
Raman scattering or the Raman effect is the inelastic scattering of photons by matter, meaning that there is both an exchange of energy and a change in the light's direction. Typically this effect involves vibrational energy being gained by a molecule as incident photons from a visible laser are shifted to lower energy. This is called normal Stokes Raman scattering. The effect is exploited by chemists and physicists to gain information about materials for a variety of purposes by performing various forms of Raman spectroscopy. Many other variants of Raman spectroscopy allow rotational energy to be examined and electronic energy levels may be examined if an X-ray source is used in addition to other possibilities. More complex techniques involving pulsed lasers, multiple laser beams and so on are known.
Resonance Raman spectroscopy is a Raman spectroscopy technique in which the incident photon energy is close in energy to an electronic transition of a compound or material under examination. The frequency coincidence can lead to greatly enhanced intensity of the Raman scattering, which facilitates the study of chemical compounds present at low concentrations.

According to quantum mechanics, atoms and molecules can only hold certain defined quantities of energy, or exist in specific states. When such quanta of electromagnetic radiation are emitted or absorbed by an atom or molecule, energy of the radiation changes the state of the atom or molecule from an initial state to a final state. An absorption band is a range of wavelengths, frequencies or energies in the electromagnetic spectrum which are characteristic of a particular transition from initial to final state in a substance.
In atomic physics, Doppler broadening is the broadening of spectral lines due to the Doppler effect caused by a distribution of velocities of atoms or molecules. Different velocities of the emitting particles result in different Doppler shifts, the cumulative effect of which is the line broadening. This resulting line profile is known as a Doppler profile. A particular case is the thermal Doppler broadening due to the thermal motion of the particles. Then, the broadening depends only on the frequency of the spectral line, the mass of the emitting particles, and their temperature, and therefore can be used for inferring the temperature of an emitting body.

Doppler cooling is a mechanism that can be used to trap and slow the motion of atoms to cool a substance. The term is sometimes used synonymously with laser cooling, though laser cooling includes other techniques.
Laser absorption spectrometry (LAS) refers to techniques that use lasers to assess the concentration or amount of a species in gas phase by absorption spectrometry (AS).
Noise-immune cavity-enhanced optical-heterodyne molecular spectroscopy (NICE-OHMS) is an ultra-sensitive laser-based absorption technique that utilizes laser light to assess the concentration or the amount of a species in gas phase by absorption spectrometry (AS).
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.
In spectroscopy, the Dicke effect, also known as Dicke narrowing or sometimes collisional narrowing, named after Robert H. Dicke, refers to narrowing of the Doppler broadening of a spectral line due to collisions the emitting species experiences with other particles.
In experimental atomic physics, saturated absorption spectroscopy or Doppler-free spectroscopy is a set-up that enables the precise determination of the transition frequency of an atom between its ground state and an optically excited state. The accuracy to which these frequencies can be determined is, ideally, limited only by the width of the excited state, which is the inverse of the lifetime of this state. However, the samples of atomic gas that are used for that purpose are generally at room temperature, where the measured frequency distribution is highly broadened due to the Doppler effect. Saturated absorption spectroscopy allows precise spectroscopy of the atomic levels without having to cool the sample down to temperatures at which the Doppler broadening is no longer relevant. It is also used to lock the frequency of a laser to the precise wavelength of an atomic transition in atomic physics experiments.
Spectral line shape describes the form of a feature, observed in spectroscopy, corresponding to an energy change in an atom, molecule or ion. This shape is also referred to as the spectral line profile. Ideal line shapes include Lorentzian, Gaussian and Voigt functions, whose parameters are the line position, maximum height and half-width. Actual line shapes are determined principally by Doppler, collision and proximity broadening. For each system the half-width of the shape function varies with temperature, pressure and phase. A knowledge of shape function is needed for spectroscopic curve fitting and deconvolution.

Resonance ionization is a process in optical physics used to excite a specific atom beyond its ionization potential to form an ion using a beam of photons irradiated from a pulsed laser light. In resonance ionization, the absorption or emission properties of the emitted photons are not considered, rather only the resulting excited ions are mass-selected, detected and measured. Depending on the laser light source used, one electron can be removed from each atom so that resonance ionization produces an efficient selectivity in two ways: elemental selectivity in ionization and isotopic selectivity in measurement.