Cyanobacteria, also called Cyanobacteriota or Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name cyanobacteria refers to their color, which similarly forms the basis of cyanobacteria's common name, blue-green algae, although they are not usually scientifically classified as algae. They appear to have originated in a freshwater or terrestrial environment. Cyanobacteria produce a range of toxins known as cyanotoxins that can cause harmful health effects in humans and animals.

Lyngbya is a genus of cyanobacteria, unicellular autotrophs that form the basis of the oceanic food chain.

Cyanotoxins are toxins produced by cyanobacteria. Cyanobacteria are found almost everywhere, but particularly in lakes and in the ocean where, under high concentration of phosphorus conditions, they reproduce exponentially to form blooms. Blooming cyanobacteria can produce cyanotoxins in such concentrations that they can poison and even kill animals and humans. Cyanotoxins can also accumulate in other animals such as fish and shellfish, and cause poisonings such as shellfish poisoning.
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative stress caused by excess or fluctuating light conditions. Humans and other animals have also developed photoprotective mechanisms to avoid UV photodamage to the skin, prevent DNA damage, and minimize the downstream effects of oxidative stress.

Biological pigments, also known simply as pigments or biochromes, are substances produced by living organisms that have a color resulting from selective color absorption. Biological pigments include plant pigments and flower pigments. Many biological structures, such as skin, eyes, feathers, fur and hair contain pigments such as melanin in specialized cells called chromatophores. In some species, pigments accrue over very long periods during an individual's lifespan.

Moorea producens is a species of filamentous cyanobacteria in the genus Moorea, including tropical marine strains formerly classified as Lyngbya majuscula due to morphological resemblance but separated based on genetic evidence. Moorea producens grows on seagrass and is one of the causes of the human skin irritation seaweed dermatitis. It is known as fireweed in Australia and stinging limu in Hawaii.

Synechococcus is a unicellular cyanobacterium that is very widespread in the marine environment. Its size varies from 0.8 to 1.5 µm. The photosynthetic coccoid cells are preferentially found in well–lit surface waters where it can be very abundant. Many freshwater species of Synechococcus have also been described.

Cyanophages are viruses that infect cyanobacteria, also known as Cyanophyta or blue-green algae. Cyanobacteria are a phylum of bacteria that obtain their energy through the process of photosynthesis. Although cyanobacteria metabolize photoautotrophically like eukaryotic plants, they have prokaryotic cell structure. Cyanophages can be found in both freshwater and marine environments. Marine and freshwater cyanophages have icosahedral heads, which contain double-stranded DNA, attached to a tail by connector proteins. The size of the head and tail vary among species of cyanophages. Cyanophages infect a wide range of cyanobacteria and are key regulators of the cyanobacterial populations in aquatic environments, and may aid in the prevention of cyanobacterial blooms in freshwater and marine ecosystems. These blooms can pose a danger to humans and other animals, particularly in eutrophic freshwater lakes. Infection by these viruses is highly prevalent in cells belonging to Synechococcus spp. in marine environments, where up to 5% of cells belonging to marine cyanobacterial cells have been reported to contain mature phage particles.
Cyanobionts are cyanobacteria that live in symbiosis with a wide range of organisms such as terrestrial or aquatic plants; as well as, algal and fungal species. They can reside within extracellular or intracellular structures of the host. In order for a cyanobacterium to successfully form a symbiotic relationship, it must be able to exchange signals with the host, overcome defense mounted by the host, be capable of hormogonia formation, chemotaxis, heterocyst formation, as well as possess adequate resilience to reside in host tissue which may present extreme conditions, such as low oxygen levels, and/or acidic mucilage. The most well-known plant-associated cyanobionts belong to the genus Nostoc. With the ability to differentiate into several cell types that have various functions, members of the genus Nostoc have the morphological plasticity, flexibility and adaptability to adjust to a wide range of environmental conditions, contributing to its high capacity to form symbiotic relationships with other organisms. Several cyanobionts involved with fungi and marine organisms also belong to the genera Richelia, Calothrix, Synechocystis, Aphanocapsa and Anabaena, as well as the species Oscillatoria spongeliae. Although there are many documented symbioses between cyanobacteria and marine organisms, little is known about the nature of many of these symbioses. The possibility of discovering more novel symbiotic relationships is apparent from preliminary microscopic observations.
Mycosporine-like amino acids (MAAs) are small secondary metabolites produced by organisms that live in environments with high volumes of sunlight, usually marine environments. The exact number of compounds within this class of natural products is yet to be determined, since they have only relatively recently been discovered and novel molecular species are constantly being discovered; however, to date their number is around 30. They are commonly described as “microbial sunscreens” although their function is believed not to be limited to sun protection. MAAs represent high potential in cosmetics, and biotechnological applications. Indeed, their UV-absorbing properties would allow to create products derived from natural photoprotectors, potentially harmless to the environment and efficient against UV damage.

Lyngbyatoxin-a is a cyanotoxin produced by certain cyanobacteria species, most notably Moorea producens. It is produced as defense mechanism to ward off any would-be predators of the bacterium, being a potent blister agent as well as carcinogen. Low concentrations cause a common skin condition known as seaweed dermatitis.

Lyngbya majuscula is a species of filamentous cyanobacteria in the genus Lyngbya. It is named after the Dane Hans Christian Lyngbye.
Cyanopeptolins (CPs) are a class of oligopeptides produced by Microcystis and Planktothrix algae strains, and can be neurotoxic. The production of cyanopeptolins occurs through nonribosomal peptides synthases (NRPS).
Curacin A is a hybrid polyketide synthase (PKS)/nonribosomal peptide synthase (NRPS) derived natural product produced isolated from the cyanobacterium Lyngbya majuscula. Curacin A belongs to a family of natural products including jamaicamide, mupirocin, and pederin that have an unusual terminal alkene. Additionally, Curacin A contains a notable thiazoline ring and a unique cyclopropyl moiety, which is essential to the compound's biological activity. Curacin A has been characterized as potent antiproliferative cytotoxic compound with notable anticancer activity for several cancer lines including renal, colon, and breast cancer. Curacin A has been shown to interact with colchicine binding sites on tubulin, which inhibits microtubule polymerization, an essential process for cell division and proliferation.

Antillatoxin (ATX) is a potent lipopeptide neurotoxin produced by the marine cyanobacterium Lyngbya majuscula. ATX activates voltage-gated sodium channels, which can cause cell depolarisation, NMDA-receptor overactivity, excess calcium influx and neuronal necrosis.

Annick Wilmotte is a Belgian Antarctic researcher, best known for her research on the diversity and ecology of Antarctic cyanobacterial microflora. A genus of Antarctic cyanobacteria, called Wilmottia was named after her in recognition of her work in this field.

Oscillatoria brevis is a species of the genus Oscillatoria first identified in 1892. It is a blue-green filamentous cyanobacterium, which can be found in brackish and fresh waterways. O. brevis can also be isolated from soil.
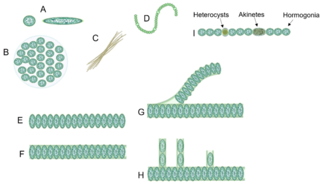
Cyanobacterial morphology refers to the form or shape of cyanobacteria. Cyanobacteria are a large and diverse phylum of bacteria defined by their unique combination of pigments and their ability to perform oxygenic photosynthesis.
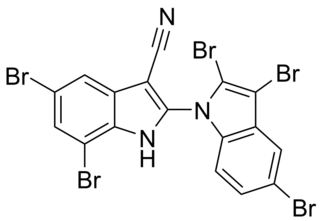
Aetokthonotoxin (AETX), colloquially known as eagle toxin, is a chemical compound that was identified in 2021 as the cyanobacterial neurotoxin causing vacuolar myelinopathy (VM) in eagles in North America. As the biosynthesis of aetokthonotoxin depends on the availability of bromide in freshwater systems and requires an interplay between the toxin-producing cyanobacterium Aetokthonos hydrillicola and the host plant it epiphytically grows on, it took more than 25 years to identify aetokthonotoxin as the VM-inducing toxin after the disease has first been diagnosed in bald eagles in 1994. The toxin cascades through the food-chain: Among other animals, it affects fish and waterfowl such as coots or ducks which feed on hydrilla colonized with the cyanobacterium. Aetokthonotoxin is transmitted to raptors, such as the bald eagle, that prey on these affected animals. The total synthesis of AETX has been achieved in 2021, the enzymatic functions of the 5 enzymes involved in AETX biosynthesis were described in 2022.
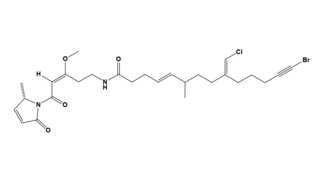
Jamaicamide A is a lipopeptide isolated from the cyanobacterium Moorea producens, formerly known as Lyngbya majuscula. Jamaicamide A belongs to a family of compounds collectively called jamaicamides, which are sodium channel blockers with potent neurotoxicity in a cellular model. Jamaicamide A has several unusual functionalities, including an alkynyl bromide, vinyl chloride, β-methoxy eneone system, and pyrrolinone ring.