
Brain abscess is an abscess within the brain tissue caused by inflammation and collection of infected material coming from local or remote infectious sources. The infection may also be introduced through a skull fracture following a head trauma or surgical procedures. Brain abscess is usually associated with congenital heart disease in young children. It may occur at any age but is most frequent in the third decade of life.

Streptococcus is a genus of gram-positive coccus or spherical bacteria that belongs to the family Streptococcaceae, within the order Lactobacillales, in the phylum Bacillota. Cell division in streptococci occurs along a single axis, so as they grow, they tend to form pairs or chains that may appear bent or twisted. This differs from staphylococci, which divide along multiple axes, thereby generating irregular, grape-like clusters of cells. Most streptococci are oxidase-negative and catalase-negative, and many are facultative anaerobes.

Streptococcus pyogenes is a species of Gram-positive, aerotolerant bacteria in the genus Streptococcus. These bacteria are extracellular, and made up of non-motile and non-sporing cocci that tend to link in chains. They are clinically important for humans, as they are an infrequent, but usually pathogenic, part of the skin microbiota that can cause Group A streptococcal infection. S. pyogenes is the predominant species harboring the Lancefield group A antigen, and is often called group A Streptococcus (GAS). However, both Streptococcus dysgalactiae and the Streptococcus anginosus group can possess group A antigen as well. Group A streptococci, when grown on blood agar, typically produce small (2–3 mm) zones of beta-hemolysis, a complete destruction of red blood cells. The name group A (beta-hemolytic) Streptococcus (GABHS) is thus also used.
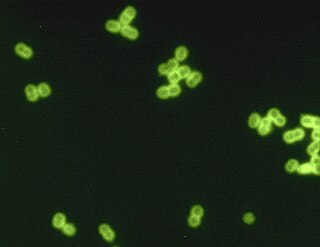
Streptococcus pneumoniae, or pneumococcus, is a Gram-positive, spherical bacteria, alpha-hemolytic member of the genus Streptococcus. They are usually found in pairs (diplococci) and do not form spores and are non motile. As a significant human pathogenic bacterium S. pneumoniae was recognized as a major cause of pneumonia in the late 19th century, and is the subject of many humoral immunity studies.

The viridans streptococci are a large group of commensal streptococcal Gram-positive bacteria species that are α-hemolytic, producing a green coloration on blood agar plates, although some species in this group are actually γ-hemolytic, meaning they produce no change on blood agar. The pseudo-taxonomic term "Streptococcus viridans" is often used to refer to this group of species, but writers who do not like to use the pseudotaxonomic term prefer the terms viridans streptococci, viridans group streptococci (VGS), or viridans streptococcal species.

Peptostreptococcus is a genus of anaerobic, Gram-positive, non-spore forming bacteria. The cells are small, spherical, and can occur in short chains, in pairs or individually. They typically move using cilia. Peptostreptococcus are slow-growing bacteria with increasing resistance to antimicrobial drugs. Peptostreptococcus is a normal inhabitant of the healthy lower reproductive tract of women.

Eikenella corrodens is a Gram-negative facultative anaerobic bacillus that can cause severe invasive disease in humans. It was first identified by M. Eiken in 1958, who called it Bacteroides corrodens. E. corrodens is a rare pericarditis associated pathogen. It is a fastidious, slow growing, human commensal bacillus, capable of acting as an opportunistic pathogen and causing abscesses in several anatomical sites, including the liver, lung, spleen, and submandibular region. E. corrodens could independently cause serious infection in both immunocompetent and immunocompromised hosts.
Streptococcus constellatus is a species of Streptococcus that is part of the normal flora in the oral cavity, urogenital region, and intestinal tract. However, it can frequently cause purulent infections in other parts of the body. DNA homology studies and 16S rRNA sequence analysis demonstrate S. constellatus belongs to the Streptococcus anginosus group along with Streptococcus intermedius and Streptococcus anginosus.

Streptococcus anginosus is a species of Streptococcus. This species, Streptococcus intermedius, and Streptococcus constellatus constitute the anginosus group, which is sometimes also referred to as the milleri group after the previously assumed but later refuted idea of a single species Streptococcus milleri. Phylogenetic relatedness of S. anginosus, S. constellatus, and S. intermedius has been confirmed by rRNA sequence analysis.

The Streptococcus anginosus group (SAG), also known as the anginosus group streptococci (AGS) or the milleri group streptococci (MGS), are a group of several species of streptococci with clinical similarities. The group is named after a principal member species, Streptococcus anginosus. The older name Streptococcus milleri is now pseudotaxonomic, as the idea that these streptococci constituted a single species was incorrect. The anginosus group streptococci are members of the viridans streptococci group. They have been implicated as etiologic agents in a variety of serious purulent infections, but because of their heterogeneous characteristics, these organisms may be unrecognized or misidentified by clinical laboratorians. The unique characteristic of them from other pathogenic streptococci, such as S. pyogenes and S. agalactiae, is their ability to cause abscesses.

Streptococcus canis is a group G beta-hemolytic species of Streptococcus. It was first isolated in dogs, giving the bacterium its name. These bacteria are characteristically different from Streptococcus dysgalactiae, which is a human-specific group G species that has a different phenotypic chemical composition. S. canis is important to the skin and mucosal health of cats and dogs, but under certain circumstances, these bacteria can cause opportunistic infections. These infections were known to afflict dogs and cats prior to the formal description of the species in Devriese et al., 1986. However, additional studies revealed cases of infection in other mammal species, including cattle and even humans. Instances of mortality from S. canis in humans are very low with only a few reported cases, while actual instances of infection may be underreported due to mischaracterizations of the bacteria as S. dysgalactiae. This species, in general, is highly susceptible to antibiotics, and plans to develop a vaccine to prevent human infections are currently being considered.

Streptococcus dysgalactiae is a gram positive, beta-haemolytic, coccal bacterium belonging to the family Streptococcaceae. It is capable of infecting both humans and animals, but is most frequently encountered as a commensal of the alimentary tract, genital tract, or less commonly, as a part of the skin flora. The clinical manifestations in human disease range from superficial skin-infections and tonsillitis, to severe necrotising fasciitis and bacteraemia. The incidence of invasive disease has been reported to be rising. Several different animal species are susceptible to infection by S. dysgalactiae, but bovine mastitis and infectious arthritis in lambs have been most frequently reported.

Streptococcus iniae is a species of Gram-positive, sphere-shaped bacterium belonging to the genus Streptococcus. Since its isolation from an Amazon freshwater dolphin in the 1970s, S. iniae has emerged as a leading fish pathogen in aquaculture operations worldwide, resulting in over US$100M in annual losses. Since its discovery, S. iniae infections have been reported in at least 27 species of cultured or wild fish from around the world. Freshwater and saltwater fish including tilapia, red drum, hybrid striped bass, and rainbow trout are among those susceptible to infection by S. iniae. Infections in fish manifest as meningoencephalitis, skin lesions, and septicemia.
Staphylococcus intermedius is a Gram-positive, catalase positive member of the bacterial genus Staphylococcus consisting of clustered cocci. Strains of this species were originally isolated from the anterior nares of pigeons, dogs, cats, mink, and horses. Many of the isolated strains show coagulase activity. Clinical tests for detection of methicillin-resistant S. aureus may produce false positives by detecting S. intermedius, as this species shares some phenotypic traits with methicillin-resistant S. aureus strains. It has been theorized that S. intermedius has previously been misidentified as S. aureus in human dog bite wound infections, which is why molecular technologies such as MALDI-TOF and PCR are preferred in modern veterinary clinical microbiology laboratories for their more accurate identifications over biochemical tests. S. intermedius is largely phenotypically indiscriminate from Staphylococcus pseudintermedius and Staphylococcus delphini, and therefore the three organisms are considered to be included in the more general 'Staphylococcus intermedius group'.
Nocardia farcinica is a species of bacteria, once thought to be associated with farcy, and a member of the genus Nocardia. This species is very similar in phenotype to Nocardia asteroides, to the degree that some isolates of N. asteroides were later found to be Nocardia farcinica.
Staphylococcus pseudintermedius is a gram positive coccus bacteria of the genus Staphylococcus found worldwide. It is primarily a pathogen for domestic animals, but has been known to affect humans as well. S. pseudintermedius is an opportunistic pathogen that secretes immune modulating virulence factors, has many adhesion factors, and the potential to create biofilms, all of which help to determine the pathogenicity of the bacterium. Diagnoses of Staphylococcus pseudintermedius have traditionally been made using cytology, plating, and biochemical tests. More recently, molecular technologies like MALDI-TOF, DNA hybridization and PCR have become preferred over biochemical tests for their more rapid and accurate identifications. This includes the identification and diagnosis of antibiotic resistant strains.
Rickettsia asiatica is a tick-borne pathogenic species borne by Ixodes ovatus. The type strain of Rickettsia asiatica sp. nov. is IO-1T.
Streptococcus infantarius is a species of bacteria.
Mycoplasma salivarium is a species of bacteria in the genus Mycoplasma. This genus of bacteria lacks a cell wall around their cell membrane. Without a cell wall, they are unaffected by many common antibiotics such as penicillin or other beta-lactam antibiotics that target cell wall synthesis. Mycoplasma are the smallest bacterial cells yet discovered, can survive without oxygen and are typically about 0. 1 μm in diameter. Mycoplasma salivarium is found in the mouths of 97% of the healthy population, and is generally considered to be a commensal organism and part of the normal oral flora.

Corynebacterium xerosis is a Gram-positive, rod-shaped bacterium in the genus Corynebacterium. Although it is frequently a harmless commensal organism living on the skin and mucus membranes, C. xerosis is also a clinically-relevant opportunistic pathogen that has been attributed to a number of different infections in animals and humans. However, its actual prominence in human medicine is up for debate due to early difficulties distinguishing it from other Corynebacterium species in clinical isolates.











