
The Pauson–Khand reaction is a chemical reaction described as a [2+2+1] cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone. Ihsan Ullah Khand (1935-1980) discovered the reaction around 1970, while working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013) at the University of Strathclyde in Glasgow. Pauson and Khand's initial findings were intermolecular in nature, but starting a decade after the reaction's discovery, many intramolecular examples have been highlighted in both synthesis and methodology reports. This reaction was originally mediated by stoichiometric amounts of dicobalt octacarbonyl, but newer versions are both more efficient, enhancing reactivity and yield via utilizing different chiral auxiliaries for stereo induction, main group transition-metals, and additives.
The Étard reaction is a chemical reaction that involves the direct oxidation of an aromatic or heterocyclic bound methyl group to an aldehyde using chromyl chloride. For example, toluene can be oxidized to benzaldehyde.
Streptomyces spectabilis is a bacterium species from the genus of Streptomyces. Streptomyces spectabilis produces hangtaimycin, gentamicin, kanamycin, neomycin B, sisomycin, tobramycin, paromomycin, spectinabilin, spectinomycin, aminocyclitol, actinospectacin, prodigiosine and the streptovaricin complex.

Desosamine is a 3-(dimethylamino)-3,4,6-trideoxyhexose found in certain macrolide antibiotics such as the commonly prescribed erythromycin, azithromycin, clarithroymcin, methymycin, narbomycin, oleandomycin, picromycin and roxithromycin. As the name suggests, these macrolide antibiotics contain a macrolide or lactone ring and they are attached to the ring Desosamine which is crucial for bactericidal activity. The biological action of the desosamine-based macrolide antibiotics is to inhibit the bacterial ribosomal protein synthesis. These antibiotics which contain Desosamine are widely used to cure bacterial-causing infections in human respiratory system, skin, muscle tissues, and urethra.

Sparsomycin is a compound, initially discovered as a metabolite of the bacterium Streptomyces sparsogenes, which binds to the 50S ribosomal subunit and inhibits protein synthesis through peptidyl transferase inhibition. As it binds to the 50S ribosomal subunit, it induces translocation on the 30S subunit. It is a nucleotide analogue. It was also formerly thought to be a possible anti-tumor agent, but interest in this drug was later discarded after it was discovered that it resulted in retinopathy and as a tool to study protein synthesis; it is not specific for bacterial ribosomes and so not usable as an antibiotic.

Callystatin A is a polyketide natural product from the leptomycin family of secondary metabolites. It was first isolated in 1997 from the marine sponge Callyspongia truncata which was collected from the Goto Islands in the Nagasaki Prefecture of Japan by the Kobayashi group. Since then its absolute configuration has been elucidated and callystatin A was discovered to have anti-fungal and anti-tumor activities with extreme potency against the human epidermoid carcinoma KB cells (IG50 = 10 pg/ml) and the mouse lymphocytic leukemia Ll210 cells (IG50 = 20 pg/ml).

Kidamycin is an anthracycline antibiotic with anticancer activity. It was first synthesized from a strain of streptomyces bacteria isolated from a soil sample. In clinical trials, Kindamycin showed high effect against gram positive bacteria as well as multiple cancer models including Ehrlich ascites carcinoma, Sarcoma 180, NF-sarcoma, and Yoshida sarcoma.
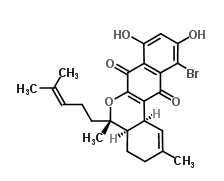
Marinone is an antibiotic made by marine actinomycetes.

The cyclothiazomycins are a group of natural products, classified as thiopeptides, which are produced by various Streptomyces species of bacteria.
Streptomyces candidus is a bacterium species from the genus of Streptomyces which has been isolated from soil in Russia. Streptomyces candidus produces lemonomycin, enterocin, pyrazofurin and avoparcin.
Streptomyces capoamus is a bacterium species from the genus of Streptomyces which has been isolated from soil from Iceland. Streptomyces capoamus produces capomycin, ciclamycin O, ciclamycin 4, anthracycline, ciclacidin A, ciclacidin B and ciclamicin.
Streptomyces halstedii is a bacterium species from the genus of Streptomyces which has been isolated from deeper soil layers. Streptomyces halstedii produces magnamycin B, vicenistatin deltamycin A2, deltamycin A3, bafilomycin B1 and bafilomycin C1. Streptomyces halstedii also produces complex antifungal antibiotics like oligomycins and the antibiotics anisomycin and sinefungin.
Streptomyces prunicolor is a bacterium species from the genus of Streptomyces which has been isolated from soil in Russia. Streptomyces prunicolor produces Pironetin and the free radical scavengers benthocyanin A, benthocyanin B and benthocyanin C.
Streptomyces spiroverticillatus is a bacterium species from the genus of Streptomyces which has been isolated from soil in Japan. Streptomyces spiroverticillatus produces tautomycin.
Streptomyces thermoviolaceus is a thermophilic bacterium species from the genus of Streptomyces which has been isolated from composts. Streptomyces thermoviolaceus produces chitinase and peroxidase.
Streptomyces violaceusniger is a bacterium species from the genus of Streptomyces. Streptomyces violaceusniger has antifungal activity. Streptomyces violaceusniger produces isoafricanol and spirofungin.
Streptomyces violascens is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces violascens produces violapyrone A – G, L-glutamate oxidase and albaflavenoid.

C-1027 or Lidamycin is an antitumor antibiotic consisting of a complex of an enediyne chromophore and an apoprotein. It shows antibiotic activity against most Gram-positive bacteria. It is one of the most potent cytotoxic molecules known, due to its induction of a higher ratio of DNA double-strand breaks than single-strand breaks.

Abikoviromycin is an antiviral antibiotic piperidine alkaloid with the molecular formula C10H11ON which is produced by the bacteria Streptomyces abikoensis and Streptomyces rubescens.

Angustmycin A is a purine antibiotic and metabolite from Streptomyces bacteria with the molecular formula C11H13N5O4. Angustmycin A is also a cytokinin.









