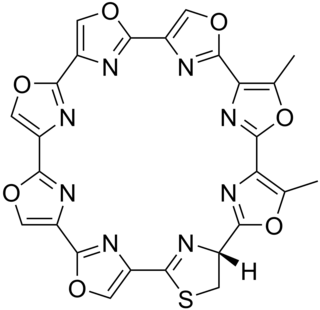
Telomestatin is a macrocyclic chemical compound that acts by inhibiting the telomerase activity of in vitro cancer cells. It was first isolated from the bacteria Streptomyces anulatus. Telomestatin induces the formation of basket-type G-quadruplex (G4) structures from hybrid-type G-quadruplexes in the telomeric region. Upon formation of G4 structure there will be a decrease in the activity of the telomerase, which is involved in the replication of the telomeres and as a result the cell dies due to Hayflick type senescence.
Terpentetriene synthase is an enzyme with systematic name terpentedienyl-diphosphate diphosphate-lyase (terpentetriene-forming). This enzyme catalyses the following chemical reaction
Terpentedienyl-diphosphate synthase is an enzyme with systematic name terpentedienyl-diphosphate lyase (decyclizing). This enzyme catalyses the following chemical reaction

The cyclothiazomycins are a group of natural products, classified as thiopeptides, which are produced by various Streptomyces species of bacteria.
Streptomyces anulatus is a bacterium species from the genus Streptomyces which has been isolated from soil. Streptomyces anulatus produces cactinomycin, endophenazine A, endophenazine B, tubermycin B, endophenazine C, epocarbazolin A, epocarbazolin B, dextranase, telomestatin and actinomycin C.
Streptomyces chartreusis is a bacterium species from the genus of Streptomyces which has been isolated from soil in Africa. Streptomyces chartreusis produces N-deacyltunicamycin, elsamicin A, aminoacylase and chartreusin.
Streptomyces cinereus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces cinereus produces ferramidochloromycin.
Streptomyces collinus is a bacterium species from the genus of Streptomyces which has been isolated from soil in Baden in Germany. Streptomyces collinus produces ansatrienin A2, ansatrienin A3, ansatrienin B, naphthomycin A, collinomycine, toromycin, streptocollin, kirromycin and rubromycine.
Streptomyces diastatochromogenes is a bacterium species from the genus of Streptomyces. Streptomyces diastatochromogenes produces polyketomycin, concanamycin A, concanamycin B, concanamycin C, momofulvenone A, azdimycin, toyocamycin and oligomycins.
Streptomyces gardneri is a bacterium species from the genus of Streptomyces. Streptomyces gardneri produces thiopeptide A, proactinomycin A, proactinomycin B, proactinomycin C.
Streptomyces halstedii is a bacterium species from the genus of Streptomyces which has been isolated from deeper soil layers. Streptomyces halstedii produces magnamycin B, vicenistatin deltamycin A2, deltamycin A3, bafilomycin B1 and bafilomycin C1. Streptomyces halstedii also produces complex antifungal antibiotics like oligomycins and the antibiotics anisomycin and sinefungin.
Streptomyces nitrosporeus is a bacterium species from the genus of Streptomyces which has been isolated from garden soil in Japan. Streptomyces nitrosporeus produces Benzastatin E, Benzastatin F, Benzastatin G Nitrosporeusine A and Nitrosporeusine B and the antibiotics nitrosporin and virantomycin and the inhibitor of angiotensin-converting enzyme foroxymithine. Streptomyces nitrosporeus can degrade cellulose.
Streptomyces phaeochromogenes is a bacterium species from the genus of Streptomyces. Streptomyces phaeochromogenes produces tyrosinate, bromoperoxidase, ditryptophenalin, phaeochromycin A, phaeochromycin B, phaeochromycin C, phaeochromycin D and phaeochromycin E. Streptomyces phaeochromogenes also produces moenomycin and bambermycin.
Kitasatospora purpeofusca is a bacterium species from the genus Kitasatospora which has been isolated from soil in Japan. Kitasatospora purpeofusca produces negamycin, aestivophoenin A, aestivophoenin B, aestivophoenin C and heptaene.
Streptomyces showdoensis is a bacterium species from the genus of Streptomyces which has been isolated from soil in Shōdoshima, Japan. Streptomyces showdoensis produces terferol, actinomycin and showdomycin.
Streptomyces wedmorensis is a bacterium species from the genus of Streptomyces which has been isolated from soil in Pennsylvania in the United States. Streptomyces wedmorensis produces (S)-2-hydroxypropylphosphonic acid epoxidase, fosfomycin and phosphonomycin B.
Streptomyces lydicamycinicus is a bacterium species from the genus of Streptomyces. Streptomyces lydicamycinicus produces the antibiotic lydicamycin.
Lydicamycin is an organic compound with the molecular formula C47H74N4O10. Lydicamycin is an antibiotic with activity against Gram-positive bacteria. The bacteria Streptomyces lydicamycinicus and Streptomyces platensis produces lydicamycin.
Quinolidomicin A1 is a 60-membered macrocyclic compound isolated from Micromonospora sp. JY16. Quinolidomicins are a class of macrolides that contain a benzoquinone chromophore as well as an immense lactone ring, which far surpasses that in monozanomycin. It is currently the largest identified macrolide of terrestrial origin. It was initially discovered when in a screening for anti-tumor antibiotics, where it was found to be cytotoxic against P388 murine leukemia cells (IC50 8 nM), and has later been found to have strong cytotoxic activity against HT-29, MKN28, K562, and KB.

Capomycin is an antitumor antibiotic with the molecular formula C35H38O10 which is produced by the bacterium Streptomyces capoamus.



