
In organic chemistry, isocyanate is the functional group with the formula R−N=C=O. Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers.
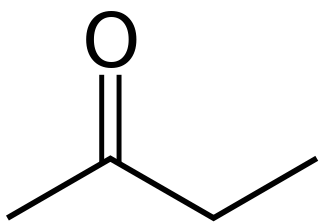
Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.

Chemical waste is any excess, unused, or unwanted chemical. Chemical waste may be classified as hazardous waste, non-hazardous waste, universal waste, or household hazardous waste, each of which is regulated separately by national governments and the United Nations. Hazardous waste is material that displays one or more of the following four characteristics: ignitability, corrosivity, reactivity, and toxicity. This information, along with chemical disposal requirements, is typically available on a chemical's Safety Data Sheet (SDS). Radioactive and biohazardous wastes require additional or different methods of handling and disposal, and are often regulated differently than standard hazardous wastes.

Dichloromethane is an organochlorine compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.

Trichloroethylene (TCE) is a halocarbon with the formula C2HCl3, commonly used as an industrial degreasing solvent. It is a clear, colourless, non-flammable, volatile liquid with a chloroform-like pleasant mild smell and sweet taste. Its IUPAC name is trichloroethene. Trichloroethylene has been sold under a variety of trade names. Industrial abbreviations include TCE, trichlor, Trike, Tricky and tri. Under the trade names Trimar and Trilene, it was used as a volatile anesthetic and as an inhaled obstetrical analgesic. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene.

Lindane, also known as gamma-hexachlorocyclohexane (γ-HCH), gammaxene, Gammallin and benzene hexachloride (BHC), is an organochlorine chemical and an isomer of hexachlorocyclohexane that has been used both as an agricultural insecticide and as a pharmaceutical treatment for lice and scabies.

Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.

White spirit (AU, UK and Ireland) or mineral spirits (US, Canada), also known as mineral turpentine (AU/NZ/ZA), turpentine substitute, and petroleum spirits, is a petroleum-derived clear liquid used as a common organic solvent in painting. There are also terms for specific kinds of white spirit, including Stoddard solvent and solvent naphtha (petroleum). White spirit is often used as a paint thinner, or as a component thereof, though paint thinner is a broader category of solvent. Odorless mineral spirits (OMS) have been refined to remove the more toxic aromatic compounds, and are recommended for applications such as oil painting.

Chemical hazards are hazards present in hazardous chemicals and hazardous materials. Exposure to certain chemicals can cause acute or long-term adverse health effects. Chemical hazards are usually classified separately from biological hazards (biohazards). Chemical hazards are classified into groups that include asphyxiants, corrosives, irritants, sensitizers, carcinogens, mutagens, teratogens, reactants, and flammables. In the workplace, exposure to chemical hazards is a type of occupational hazard. The use of personal protective equipment may substantially reduce the risk of adverse health effects from contact with hazardous materials.

The Toxic Substances Control Act (TSCA) is a United States law, passed by the 94th United States Congress in 1976 and administered by the United States Environmental Protection Agency (EPA), that regulates chemicals not regulated by other U.S. federal statutes, including chemicals already in commerce and the introduction of new chemicals. When the TSCA was put into place, all existing chemicals were considered to be safe for use and subsequently grandfathered in. Its three main objectives are to assess and regulate new commercial chemicals before they enter the market, to regulate chemicals already existing in 1976 that posed an "unreasonable risk of injury to health or the environment", as for example PCBs, lead, mercury and radon, and to regulate these chemicals' distribution and use.

Bromoform is an organic compound with the chemical formula CHBr3. It is a colorless liquid at room temperature, with a high refractive index and a very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. It is a brominated organic solvent. Currently its main use is as a laboratory reagent. It is very slightly soluble in water and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone and oils.

Chloroprene (IUPAC name 2-chlorobuta-1,3-diene) is a chemical compound with the molecular formula CH2=CCl−CH=CH2. Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene, better known as neoprene, a type of synthetic rubber.

Environmental hazards are those hazards that affect biomes or ecosystems. Well known examples include oil spills, water pollution, slash and burn deforestation, air pollution, ground fissures, and build-up of atmospheric carbon dioxide. Physical exposure to environmental hazards is usually involuntary

2-Butoxyethanol is an organic compound with the chemical formula BuOC2H4OH. This colorless liquid has a sweet, ether-like odor, as it derives from the family of glycol ethers, and is a butyl ether of ethylene glycol. As a relatively nonvolatile, inexpensive solvent, it is used in many domestic and industrial products because of its properties as a surfactant. It is a known respiratory irritant and can be acutely toxic, but animal studies did not find it to be mutagenic, and no studies suggest it is a human carcinogen. A study of 13 classroom air contaminants conducted in Portugal reported a statistically significant association with increased rates of nasal obstruction and a positive association below the level of statistical significance with a higher risk of obese asthma and increased body mass index.

1-Bromopropane (n-propylbromide or nPB) is an organobromine compound with the chemical formula CH3CH2CH2Br. It is a colorless liquid that is used as a solvent. It has a characteristic hydrocarbon odor. Its industrial applications increased dramatically in the 21st century due to the phasing out of chlorofluorocarbons and chloroalkanes such as 1,1,1-Trichloroethane under the Montreal Protocol.
The Omega Chemical Corporation was a refrigerant and solvent recycling company that operated from 1976 to 1991 in Whittier, California. Due to improper waste handling and removal, the soil and groundwater beneath the property became contaminated and the area is now referred to as the Omega Chemical Superfund Site. Cleanup of the site began in 1995 with the removal of hazardous waste receptacles and a multimillion-dollar soil vaporization detoxifying system.

Pesticide regulation in the United States is primarily a responsibility of the Environmental Protection Agency (EPA). In America, it was not till the 1950s that pesticides were regulated in terms of their safety. The Pesticides Control Amendment (PCA) of 1954 was the first time Congress passed guidance regarding the establishment of safe limits for pesticide residues on food. It authorized the Food and Drug Administration (FDA) to ban pesticides they determined to be unsafe if they were sprayed directly on food. The Food Additives Amendment, which included the Delaney Clause, prohibited the pesticide residues from any carcinogenic pesticides in processed food. In 1959, pesticides were required to be registered.
Chemical safety includes all those policies, procedures and practices designed to minimize the risk of exposure to potentially hazardous chemicals. This includes the risks of exposure to persons handling the chemicals, to the surrounding environment, and to the communities and ecosystems within that environment. Manufactured chemicals, either pure or in mixtures, solutions and emulsions, are ubiquitous in modern society, at industrial, occupational and private scale. However, there are chemicals that should not mix or get in contact with others, as they can produce byproducts that may be toxic, carcinogenic, explosive etc., or can be dangerous in themselves. To avoid disasters and mishaps, maintaining safety is paramount.
Hazard substitution is a hazard control strategy in which a material or process is replaced with another that is less hazardous. Substitution is the second most effective of the five members of the hierarchy of hazard controls in protecting workers, after elimination. Substitution and elimination are most effective early in the design process, when they may be inexpensive and simple to implement, while for an existing process they may require major changes in equipment and procedures. The concept of prevention through design emphasizes integrating the more effective control methods such as elimination and substitution early in the design phase.
Sharkey Landfill is a 90-acre property located in New Jersey along the Rockaway and Whippany rivers in Parsippany, New Jersey. Landfill operations began in 1945, and continued until September 1972, when large amounts of toluene, benzene, chloroform, dichloroethylene, and methylene chloride were found, all of which have are a hazard to human health causing cancer and organ failure. Sharkey Landfill was put on the National Priority List in 1983, and clean up operations ran until the site was deemed as not a threat in 2004.















