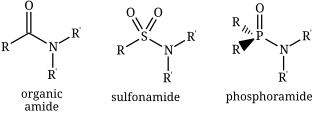
Porphyrins are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent porphyrin is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons, of which 18 π-electrons form a planar, continuous cycle, the porphyrin ring structure is often described as aromatic. One result of the large conjugated system is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives from the Greek word πορφύρα (porphyra), meaning purple.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Several isomers are possible, each of which can be viewed as a quinone derivative. The term anthraquinone, however, almost invariably refers to one specific isomer, 9,10-anthraquinone wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. For instance, it is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol.
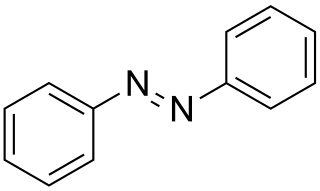
Azobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes.
The term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, halogen bonding, electrostatic and/or electromagnetic effects. In addition to these direct interactions as well solvent can play a dominant indirect role in driving molecular recognition in solution. The host and guest involved in molecular recognition exhibit molecular complementarity.

Self-assembled monolayers (SAM) of organic molecules are molecular assemblies formed spontaneously on surfaces by adsorption and are organized into more or less large ordered domains. In some cases molecules that form the monolayer do not interact strongly with the substrate. This is the case for instance of the two-dimensional supramolecular networks of e.g. perylenetetracarboxylic dianhydride (PTCDA) on gold or of e.g. porphyrins on highly oriented pyrolitic graphite (HOPG). In other cases the molecules possess a head group that has a strong affinity to the substrate and anchors the molecule to it. Such a SAM consisting of a head group, tail and functional end group is depicted in Figure 1. Common head groups include thiols, silanes, phosphonates, etc.

A supramolecular assembly or "supermolecule" is a well defined complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules, it is more often used to denote larger complexes of molecules that form sphere-, rod-, or sheet-like species. Micelles, liposomes and biological membranes are examples of supramolecular assemblies. The dimensions of supramolecular assemblies can range from nanometers to micrometers. Thus they allow access to nanoscale objects using a bottom-up approach in far fewer steps than a single molecule of similar dimensions.

Macrocycles are often described as molecules and ions containing twelve or more membered ring. Classical examples include the crown ethers, calixarenes, porphyrins, cyclodextrins. Macrocycles describe a large, mature area of chemistry.

In chemistry, a salt bridge is a combination of two non-covalent interactions: hydrogen bonding and ionic bonding. Ion pairing is one of the most important noncovalent forces in chemistry, in biological systems, in different materials and in many applications such as ion pair chromatographyga. It is a most commonly observed contribution to the stability to the entropically unfavorable folded conformation of proteins. Although noncovalent interactions are known to be relatively weak interactions, small stabilizing interactions can add up to make an important contribution to the overall stability of a conformer. Not only are salt bridges found in proteins, but they can also be found in supramolecular chemistry. The thermodynamics of each are explored through experimental procedures to assess the free energy contribution of the salt bridge to the overall free energy of the state.

In chemistry, pi stacking refers to attractive, noncovalent interactions between aromatic rings, since they contain pi bonds. These interactions are important in nucleobase stacking within DNA and RNA molecules, protein folding, template-directed synthesis, materials science, and molecular recognition, although new research suggests that pi stacking may not be operative in some of these applications. Despite intense experimental and theoretical interest, there is no unified description of the factors that contribute to pi stacking interactions.
A resorcinarene is a macrocycle, or a cyclic oligomer, based on the condensation of resorcinol (1,3-dihydroxybenzene) and an aldehyde. Resorcinarenes are a type of calixarene.

Cation–π interaction is a noncovalent molecular interaction between the face of an electron-rich π system (e.g. benzene, ethylene, acetylene) and an adjacent cation (e.g. Li+, Na+). This interaction is an example of noncovalent bonding between a monopole (cation) and a quadrupole (π system). Bonding energies are significant, with solution-phase values falling within the same order of magnitude as hydrogen bonds and salt bridges. Similar to these other non-covalent bonds, cation–π interactions play an important role in nature, particularly in protein structure, molecular recognition and enzyme catalysis. The effect has also been observed and put to use in synthetic systems.

In chemistry, aurophilicity refers to the tendency of gold complexes to aggregate via formation of weak metallophilic interactions.
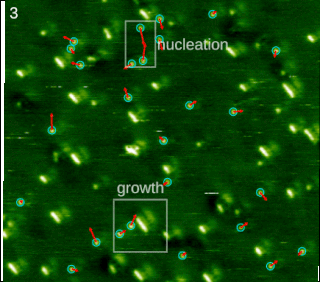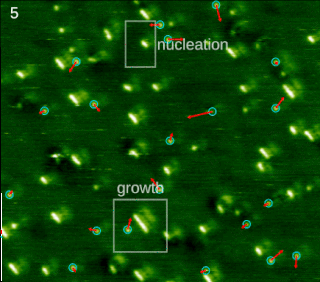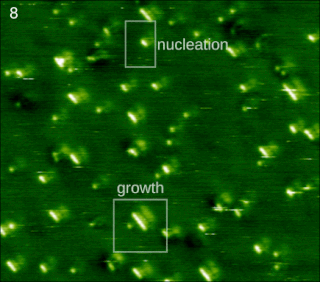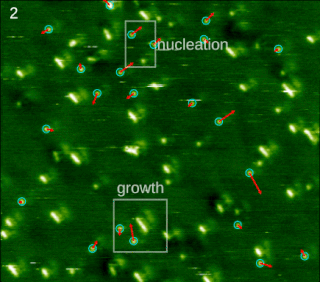
Molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly. These are intramolecular self-assembly and intermolecular self-assembly. Commonly, the term molecular self-assembly refers to intermolecular self-assembly, while the intramolecular analog is more commonly called folding.

Professor Noel Sydney Hush AO, DSc, FRS, FNAS, FAA, FRACI, FRSN is an Australian chemist at the University of Sydney.
Coordination cages are three-dimensional ordered structures in solution that act as hosts in host–guest chemistry. They are self-assembled in solution from organometallic precursors, and often rely solely on noncovalent interactions rather than covalent bonds. Coordinate bonds are useful in such supramolecular self-assembly because of their versatile geometries. However, there is controversy over calling coordinate bonds noncovalent, as they are typically strong bonds and have covalent character. The combination of a coordination cage and a guest is a type of inclusion compound. Coordination complexes can be used as "nano-laboratories" for synthesis, and to isolate interesting intermediates. The inclusion complexes of a guest inside a coordination cage show intriguing chemistry as well; often, the properties of the cage will change depending on the guest. Coordination complexes are molecular moieties, so they are distinct from clathrates and metal-organic frameworks.
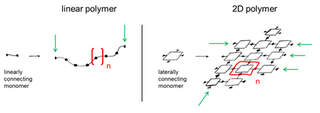
A two-dimensional polymer (2DP) is a sheet-like monomolecular macromolecule consisting of laterally connected repeat units with end groups along all edges. This recent definition of 2DP is based on Hermann Staudinger's polymer concept from the 1920s. According to this, covalent long chain molecules ("Makromoleküle") do exist and are composed of a sequence of linearly connected repeat units and end groups at both termini.
Ayyappanpillai Ajayagosh is an organic chemist, academic and the director of the National Institute for Interdisciplinary Science and Technology. He is known for his studies on supramolecular assemblies and light induced sensor systems and is an elected fellow of all the three major Indian science academies viz. the National Academy of Sciences, India, Indian National Science Academy and the Indian Academy of Sciences as well as The World Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards for his contributions to Chemical Sciences in 2007. He received the TWAS Prize of The World Academy of Sciences in 2013.
Metallogels are one-dimensional nanostructured materials, which constitute a growing class in the Supramolecular chemistry field. Non-covalent interactions, such as hydrophobic interactions, π-π interactions, and hydrogen bonding, are among the responsible forces for the formation of those gels from small molecules. However, the main driving force for the formation of a metallogel is the metal-ligand coordination. Once the structure has been established, it resists gravitational force when inverted.





![A titin-mimicking supramolecular polymers, with quadruple hydrogen bonding between 2-ureido-4[1H]-pyrimidone (UPy) moieties. Fig 2. A titin-mimicking supramolecular polymers.png](http://upload.wikimedia.org/wikipedia/commons/9/9a/Fig_2._A_titin-mimicking_supramolecular_polymers.png)