
T-box transcription factor T, also known as Brachyury protein, is encoded for in humans by the TBXT gene. Brachyury functions as a transcription factor within the T-box family of genes. Brachyury homologs have been found in all bilaterian animals that have been screened, as well as the freshwater cnidarian Hydra.

Zinc finger protein GLI3 is a protein that in humans is encoded by the GLI3 gene.

Myogenesis is the formation of skeletal muscular tissue, particularly during embryonic development.
Limb development in vertebrates is an area of active research in both developmental and evolutionary biology, with much of the latter work focused on the transition from fin to limb.
The limb bud is a structure formed early in vertebrate limb development. As a result of interactions between the ectoderm and underlying mesoderm, formation occurs roughly around the fourth week of development. In the development of the human embryo the upper limb bud appears in the third week and the lower limb bud appears four days later.
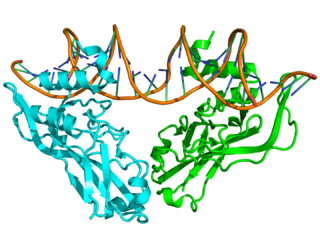
T-box refers to a group of transcription factors involved in embryonic limb and heart development. Every T-box protein has a relatively large DNA-binding domain, generally comprising about a third of the entire protein that is both necessary and sufficient for sequence-specific DNA binding. All members of the T-box gene family bind to the "T-box", a DNA consensus sequence of TCACACCT.
T-box transcription factor TBX5, is a protein that in humans is encoded by the TBX5 gene.

Fibroblast growth factor 8(FGF-8) is a protein that in humans is encoded by the FGF8 gene.

T-box transcription factor TBX3 is a protein that in humans is encoded by the TBX3 gene.
T-box transcription factor 2 Tbx2 is a transcription factor that is encoded by the Tbx2 gene on chromosome 17q21-22 in humans. This gene is a member of a phylogenetically conserved family of genes that share a common DNA-binding domain, the T-box. Tbx2 and Tbx3 are the only T-box transcription factors that act as transcriptional repressors rather than transcriptional activators, and are closely related in terms of development and tumorigenesis. This gene plays a significant role in embryonic and fetal development through control of gene expression, and also has implications in various cancers. Tbx2 is associated with numerous signaling pathways, BMP, TGFβ, Wnt, and FGF, which allow for patterning and proliferation during organogenesis in fetal development.

Paired related homeobox 1 is a protein that in humans is encoded by the PRRX1 gene.

Protein odd-skipped-related 1 is a transcription factor that in humans is encoded by the OSR1 gene. The OSR1 and OSR2 transcription factors participate in the normal development of body parts such as the kidney.

T-box transcription factor TBX22 is a protein that in humans is encoded by the TBX22 gene.

Homeobox protein goosecoid(GSC) is a homeobox protein that is encoded in humans by the GSC gene. Like other homeobox proteins, goosecoid functions as a transcription factor involved in morphogenesis. In Xenopus, GSC is thought to play a crucial role in the phenomenon of the Spemann-Mangold organizer. Through lineage tracing and timelapse microscopy, the effects of GSC on neighboring cell fates could be observed. In an experiment that injected cells with GSC and observed the effects of uninjected cells, GSC recruited neighboring uninjected cells in the dorsal blastopore lip of the Xenopus gastrula to form a twinned dorsal axis, suggesting that the goosecoid protein plays a role in the regulation and migration of cells during gastrulation.

NK3 homeobox 2 also known as NKX3-2 is a human gene. It is a homolog of bagpipe (bap) in Drosophila and therefore also known as Bapx1. The protein encoded by this gene is a homeodomain containing transcription factor.

Mesoderm posterior protein 2 (MESP2), also known as class C basic helix-loop-helix protein 6 (bHLHc6), is a protein that in humans is encoded by the MESP2 gene.
Krüppel-like factor 15 is a protein that in humans is encoded by the KLF15 gene in the Krüppel-like factor family. Its former designation KKLF stands for kidney-enriched Krüppel-like factor.

T-box 6 is a protein that in humans is encoded by the TBX6 gene.
T-box transcription factor Tbx4 is a transcription factor that belongs to T-box gene family that is involved in the regulation of embryonic developmental processes. The transcription factor is encoded by the TBX4 gene located on human chromosome 17. Tbx4 is known mostly for its role in the development of the hindlimb, but it also plays a critical role in the formation of the umbilicus. Tbx4 has been shown to be expressed in the allantois, hindlimb, lung and proctodeum.

Beta-1,3-N-acetylglucosaminyltransferase radical fringe, also known as radical fringe is a protein that in humans is encoded by the RFNG gene. Radical fringe is a signaling enzyme involved in the arrangement of the embryonic limb buds. It is a member of the fringe gene family, which also includes manic fringe and lunatic fringe. It is important for the dorsoventrally patterning of the limb and has been implicated in the formation of the apical ectodermal ridge (AER). The AER is essential for the distal patterning of the limb. Experiments executed in chicken models show Radical fringe is expressed in both the dorsal ectoderm and the AER. This provides evidence that the AER forms from cells already expressing radical fringe, though further evidence is needed to confirm. Grafting experiments have shown that formation of the AER comes from signals in the limb bud mesoderm. However, radical fringe acts as a permissive signal to create a boundary for the AER to form. The knockout experiments done in chicken models suggest Radical fringe plays an integral role in wing development.





















