
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.
Hydrolysis is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.

Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula B(OH)3. It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salts, and can react with alcohols to form borate esters.
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate BO3−3, metaborate BO−2, or tetraborate B4O2−7; or any salt of such anions, such as sodium metaborate, Na+[BO2]− and borax (Na+)2[B4O7]2−. The name also refers to esters of such anions, such as trimethyl borate B(OCH3)3.

Borax is a salt, a hydrated or anhydrous borate of sodium, with the chemical formula Na2H20B4O17. It is a colorless crystalline solid, that dissolves in water to make a basic solution.

In chemistry, there are three definitions in common use of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.
Sodium borate is a generic name for any salt of sodium with an anion consisting of boron and oxygen, and possibly hydrogen, or any hydrate thereof. It can be seen as a hydrated sodium salt of the appropriate boroxy acid, although the latter may not be a stable compound.

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.

Xanthate usually refers to a salt of xanthic acid. The formula of the salt of xanthic acid is [R−O−CS2]−M+ ,. Xanthate also refers to the anion [R−O−CS2]−. Xanthate also may refer to an ester of xanthic acid. The formula of xanthic acid is R−O−C(=S)−S−H, while the formula of the esters of xanthic acid is R−O−C(=S)−S−R', where R and R' are organyl groups. The salts of xanthates are also called O-organyl dithioates. The esters of xanthic acid are also called O,S-diorganyl esters of dithiocarbonic acid. The name xanthate is derived from Ancient Greek ξανθός xanthos, meaning “yellowish, golden”, and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and for extraction of certain sulphide bearing ores. They are also versatile intermediates in organic synthesis.
A salt metathesis reaction, sometimes called a double displacement reaction, is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding affiliations. This reaction is represented by the general scheme:

In chemistry, tetraborate or pyroborate is an anion with formula B4O2−7; or a salt containing that anion, such as sodium tetraborate, Na2B4O7. It is one of the boron oxoacids, that is, a borate.
Sodium perborate is chemical compound whose chemical formula may be written NaH2BO4, Na2H4B2O8, or, more properly, [Na+]2[B2O4(OH)4]2−. Its name is sometimes abbreviated as PBS.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Sodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder.
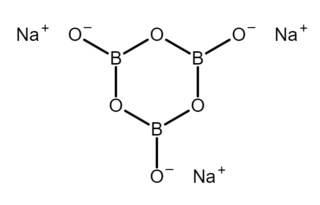
Sodium metaborate is a chemical compound of sodium, boron, and oxygen with formula NaBO2. However, the metaborate ion is trimeric in the anhydrous solid, therefore a more correct formula is Na3B3O6 or (Na+)3[B3O6]3−. The formula can be written also as Na2O·B2O3 to highlight the relation to the main oxides of sodium and boron. The name is also applied to several hydrates whose formulas can be written NaBO2·nH2O for various values of n.
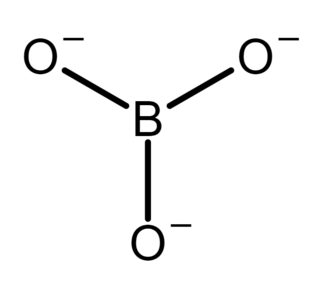
In inorganic chemistry, an orthoborate is a polyatomic anion with formula [BO3]3− or a salt containing the anion; such as trisodium orthoborate (Na+)3[BO3]3−. It is one of several boron oxyanions, or borates.
Trisodium borate is a chemical compound of sodium, boron, and oxygen, with formula Na3BO3, or (Na+)3[BO3]3−. It is a sodium salt of the orthoboric acid B(OH)3.

Sodium tetrahydroxyborate is a salt of with chemical formula NaH4BO4 or Na+[B(OH)4]−. It is one of several sodium borates. At room temperature it is a colorless crystalline solid.
















