
In organic chemistry, isothiocyanate is the functional group −N=C=S, formed by substituting the oxygen in the isocyanate group with a sulfur. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also known as mustard oils. An artificial isothiocyanate, phenyl isothiocyanate, is used for amino acid sequencing in the Edman degradation.

Allyl isothiocyanate (AITC) is an organosulfur compound (formula CH2CHCH2NCS). The colorless oil is responsible for the pungent taste of mustard, radish, horseradish, and wasabi. This pungency and the lachrymatory effect of AITC are mediated through the TRPA1 and TRPV1 ion channels. It is slightly soluble in water, but more soluble in most organic solvents.

Glucosinolates are natural components of many pungent plants such as mustard, cabbage, and horseradish. The pungency of those plants is due to mustard oils produced from glucosinolates when the plant material is chewed, cut, or otherwise damaged. These natural chemicals most likely contribute to plant defence against pests and diseases, and impart a characteristic bitter flavor property to cruciferous vegetables.

Guanidinium thiocyanate(GTC) or guanidinium isothiocyanate (GITC) is a chemical compound used as a general protein denaturant, being a chaotropic agent, although it is most commonly used as a nucleic acid protector in the extraction of DNA and RNA from cells.
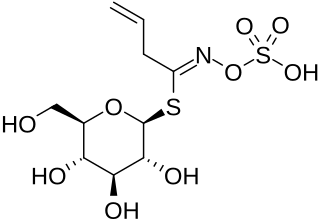
Sinigrin or allyl glucosinolate is a glucosinolate that belongs to the family of glucosides found in some plants of the family Brassicaceae such as Brussels sprouts, broccoli, and the seeds of black mustard. Whenever sinigrin-containing plant tissue is crushed or otherwise damaged, the enzyme myrosinase degrades sinigrin to a mustard oil, which is responsible for the pungent taste of mustard and horseradish. Seeds of white mustard, Sinapis alba, give a less pungent mustard because this species contains a different glucosinolate, sinalbin.

Potassium thiocyanate is the chemical compound with the molecular formula KSCN. It is an important salt of the thiocyanate anion, one of the pseudohalides. The compound has a low melting point relative to most other inorganic salts.

Glucobrassicin is a type of glucosinolate that can be found in almost all cruciferous plants, such as cabbages, broccoli, mustards, and woad. As for other glucosinolates, degradation by the enzyme myrosinase is expected to produce an isothiocyanate, indol-3-ylmethylisothiocyanate. However, this specific isothiocyanate is expected to be highly unstable, and has indeed never been detected. The observed hydrolysis products when isolated glucobrassicin is degraded by myrosinase are indole-3-carbinol and thiocyanate ion, which are envisioned to result from a rapid reaction of the unstable isothiocyanate with water. However, a large number of other reaction products are known, and indole-3-carbinol is not the dominant degradation product when glucosinolate degradation takes place in crushed plant tissue or in intact plants.

Rhodanese, also known as rhodanase, thiosulfate sulfurtransferase, thiosulfate cyanide transsulfurase, and thiosulfate thiotransferase, is a mitochondrial enzyme that detoxifies cyanide (CN−) by converting it to thiocyanate (SCN−).

Myrosinase is a family of enzymes involved in plant defense against herbivores, specifically the mustard oil bomb. The three-dimensional structure has been elucidated and is available in the PDB.
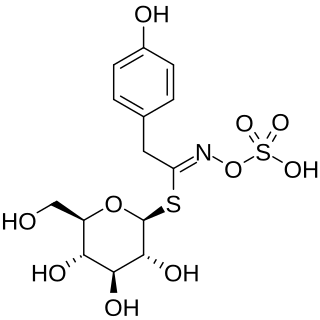
Sinalbin is a glucosinolate found in the seeds of white mustard, Sinapis alba, and in many wild plant species. In contrast to mustard from black mustard seeds which contain sinigrin, mustard from white mustard seeds has only a weakly pungent taste.
In enzymology, an aconitate Δ-isomerase is an enzyme that catalyzes the chemical reaction
In enzymology, a cholestenol Δ-isomerase is an enzyme that catalyzes the chemical reaction
In enzymology, a L-dopachrome isomerase is an enzyme that catalyzes the chemical reaction
In enzymology, a L-rhamnose isomerase is an enzyme that catalyzes the chemical reaction
In enzymology, a mannose isomerase is an enzyme that catalyzes the chemical reaction
In enzymology, a methylitaconate Δ-isomerase is an enzyme that catalyzes the chemical reaction
In enzymology, an oxaloacetate tautomerase is an enzyme that catalyzes the chemical reaction
In enzymology, a vinylacetyl-CoA Delta-isomerase is an enzyme that catalyzes the chemical reaction

Glucotropaeolin or benzyl glucosinolate is a glucosinolate found in cruciferous vegetables, particularly garden cress. Upon enzymatic activity, it is transformed into benzyl isothiocyanate, which contributes to the characteristic flavor of these brassicas.

Organic thiocyanates are organic compounds containing the functional group RSCN. the organic group is attached to sulfur: R−S−C≡N has a S–C single bond and a C≡N triple bond.












