Tight junctions, also known as occluding junctions or zonulae occludentes, are multiprotein junctional complexes whose canonical function is to prevent leakage of solutes and water and seals between the epithelial cells. They also play a critical role maintaining the structure and permeability of endothelial cells. Tight junctions may also serve as leaky pathways by forming selective channels for small cations, anions, or water. The corresponding junctions that occur in invertebrates are septate junctions.

Adherens junctions are protein complexes that occur at cell–cell junctions, cell–matrix junctions in epithelial and endothelial tissues, usually more basal than tight junctions. An adherens junction is defined as a cell junction whose cytoplasmic face is linked to the actin cytoskeleton. They can appear as bands encircling the cell or as spots of attachment to the extracellular matrix . Adherens junctions uniquely disassemble in uterine epithelial cells to allow the blastocyst to penetrate between epithelial cells.

Occludin is an enzyme that oxidizes NADH. It was first identified in epithelial cells as a 65 kDa integral plasma-membrane protein localized at the tight junctions. Together with Claudins, and zonula occludens-1 (ZO-1), occludin has been considered a staple of tight junctions, and although it was shown to regulate the formation, maintenance, and function of tight junctions, its precise mechanism of action remained elusive and most of its actions were initially attributed to conformational changes following selective phosphorylation, and its redox-sensitive dimerization. However, mounting evidence demonstrated that occludin is not only present in epithelial/endothelial cells, but is also expressed in large quantities in cells that do not have tight junctions but have very active metabolism: pericytes, neurons and astrocytes, oligodendrocytes, dendritic cells, monocytes/macrophages lymphocytes, and myocardium. Recent work, using molecular modeling, supported by biochemical and live-cell experiments in human cells demonstrated that occludin is a NADH oxidase that influences critical aspects of cell metabolism like glucose uptake, ATP production and gene expression. Furthermore, manipulation of occludin content in human cells is capable of influencing the expression of glucose transporters, and the activation of transcription factors like NFkB, and histone deacetylases like sirtuins, which proved capable of diminishing HIV replication rates in infected human macrophages under laboratory conditions.

Plakoglobin, also known as junction plakoglobin or gamma-catenin, is a protein that in humans is encoded by the JUP gene. Plakoglobin is a member of the catenin protein family and homologous to β-catenin. Plakoglobin is a cytoplasmic component of desmosomes and adherens junctions structures located within intercalated discs of cardiac muscle that function to anchor sarcomeres and join adjacent cells in cardiac muscle. Mutations in plakoglobin are associated with arrhythmogenic right ventricular dysplasia.

Alpha-catenin functions as the primary protein link between cadherins and the actin cytoskeleton. It has been reported that the actin binding proteins vinculin and alpha-actinin can bind to alpha-catenin. It has been suggested that alpha-catenin does not bind with high affinity to both actin filaments and the E-cadherin-beta-catenin complex at the same time. It has been observed that when alpha-catenin is not in a molecular complex with beta-catenin, it dimerizes and functions to regulate actin filament assembly, possibly by competing with Arp2/3 protein. Alpha catenin exhibits significant protein dynamics. However, a protein complex including a cadherin, actin, beta-catenin and alpha-catenin has not been isolated.

Afadin is a protein that in humans is encoded by the AFDN gene.

Claudin-1 is a protein that in humans is encoded by the CLDN1 gene. It belongs to the group of claudins.
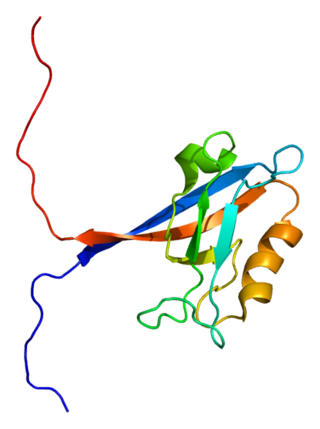
Tight junction protein ZO-2 is a protein that in humans is encoded by the TJP2 gene.

Claudin 4, also known as CLDN4, is a protein which in humans is encoded by the CLDN4 gene. It belongs to the group of claudins.

Ras GTPase-activating-like protein IQGAP1 (IQGAP1) also known as p195 is a ubiquitously expressed protein that in humans is encoded by the IQGAP1 gene. IQGAP1 is a scaffold protein involved in regulating various cellular processes ranging from organization of the actin cytoskeleton, transcription, and cellular adhesion to regulating the cell cycle.
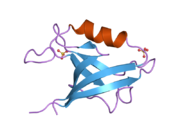
Junctional adhesion molecule A is a protein that in humans is encoded by the F11R gene. It has also been designated as CD321.

Claudin-5 is a protein that in humans is encoded by the CLDN5 gene. It belongs to the group of claudins.

Claudin 3, also known as CLDN3, is a protein which in humans is encoded by the CLDN3 gene. It is a member of the claudin protein family.

Claudin-2 is a protein that in humans is encoded by the CLDN2 gene. It belongs to the group of claudins.
InaD-like protein is a protein that in humans is encoded by the PATJ gene.

Cingulin is a cytosolic protein encoded by the CGN gene in humans localized at tight junctions (TJs) of vertebrate epithelial and endothelial cells.

Stress fibers are contractile actin bundles found in non-muscle cells. They are composed of actin (microfilaments) and non-muscle myosin II (NMMII), and also contain various crosslinking proteins, such as α-actinin, to form a highly regulated actomyosin structure within non-muscle cells. Stress fibers have been shown to play an important role in cellular contractility, providing force for a number of functions such as cell adhesion, migration and morphogenesis.

αE-catenin, also known as Catenin alpha-1 is a protein that in humans is encoded by the CTNNA1 gene. αE-catenin is highly expressed in cardiac muscle and localizes to adherens junctions at intercalated disc structures where it functions to mediate the anchorage of actin filaments to the sarcolemma. αE-catenin also plays a role in tumor metastasis and skin cell function.

Tight junction protein ZO-3 is a protein that in humans is encoded by the TJP3 gene.

Cingulin-like protein 1, also known as paracingulin or junction-associated-coiled-coil protein (JACOP), is a protein which is encoded by the CGNL1 gene.