
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.
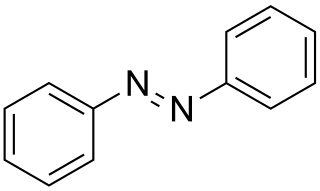
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Different classes of azo dyes exist, most notably the ones substituted with heteroaryl rings.

Azo compounds are organic compounds bearing the functional group diazenyl.

Methyl yellow, or C.I. 11020, is an organic compound with the formula C6H5N2C6H4N(CH3)2. It is an azo dye derived from dimethylaniline. It is a yellow solid. According to X-ray crystallography, the C14N3 core of the molecule is planar.

Sudan I is an organic compound typically classified as an azo dye. It is an orange-red solid, used to color waxes, oils, petrol, solvents, and polishes. Historically, Sudan I used to serve as a food coloring agent, notably for curry powder and chili powder. However, along with its derivatives Sudan III and Sudan IV, the compound has been banned in many countries due to its classification as a category 3 carcinogenic hazard by the International Agency for Research on Cancer. Nevertheless, Sudan I remains valuable as a coloring reagent for non-food-related uses, such as in the formulation of orange-colored smoke.

Quinacridone is an organic compound used as a pigment. Numerous derivatives constitute the quinacridone pigment family, which finds extensive use in industrial colorant applications such as robust outdoor paints, inkjet printer ink, tattoo inks, artists' watercolor paints, and color laser printer toner. As pigments, the quinacridones are insoluble. The development of this family of pigments supplanted the alizarin dyes.
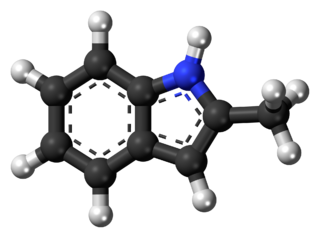
Methylketol or 2-methylindole is a mildly toxic and slightly flammable organic compound which occurs as a white solid which turns brown over time. It has chemical formula C9H9N.

Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N=N-C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60-70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.
In organic chemistry, an azo coupling is an reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activated carbon, serves as a nucleophile. Classical coupling agents are phenols and naphthols. Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate.
Pyrazolone is 5-membered heterocycle containing two adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional carbonyl (C=O) group. Compounds containing this functional group are useful commercially in analgesics and dyes.

Toluidine blue, also known as TBO or tolonium chloride (INN) is a blue cationic (basic) dye used in histology and sometimes clinically.
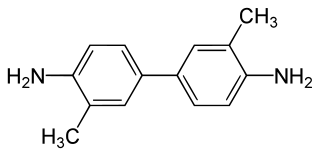
2-Tolidine (orthotolidine, o-tolidine; not to be confused with o-toluidine) is an organic compound with the chemical formula (C6H4(CH3)NH2)2. Several isomers are known; the 3-tolidine derivative is also important commercially. It is a colorless compound although commercial samples are often colored. It is slightly soluble in water. It forms salts with acids, such as the hydrochloride, which is commercially available.

Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry.

o-Anisidine (2-anisidine) is an organic compound with the formula CH3OC6H4NH2. A colorless liquid, commercial samples can appear yellow owing to air oxidation. It is one of three isomers of the methoxy-containing aniline derivative.

Acetoacetanilide is an organic compound with the formula CH3C(O)CH2C(O)NHC6H5. It is the acetoacetamide derivative of aniline. It is a white solid that is poorly soluble in water. This chemical and many related compounds (prepared from various aniline derivatives) are used in the production of organic pigments called arylide yellows. Acetoacetanilides usually exist as the keto-amide tautomer according to X-ray crystallography.
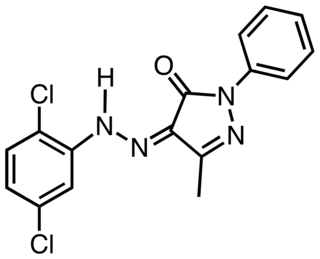
Pigment Yellow 10 is an organic compound that is classified as a monoazopyrazolone pigment. It is used as a yellow colorant, notably as yellow road marking on highways in the US.

A colorant is any substance that changes the spectral transmittance or reflectance of a material. Synthetic colorants are those created in a laboratory or industrial setting. The production and improvement of colorants was a driver of the early synthetic chemical industry, in fact many of today's largest chemical producers started as dye-works in the late 19th or early 20th centuries, including Bayer AG(1863). Synthetics are extremely attractive for industrial and aesthetic purposes as they have they often achieve higher intensity and color fastness than comparable natural pigments and dyes used since ancient times. Market viable large scale production of dyes occurred nearly simultaneously in the early major producing countries Britain (1857), France (1858), Germany (1858), and Switzerland (1859), and expansion of associated chemical industries followed. The mid-nineteenth century through WWII saw an incredible expansion of the variety and scale of manufacture of synthetic colorants. Synthetic colorants quickly became ubiquitous in everyday life, from clothing to food. This stems from the invention of industrial research and development laboratories in the 1870s, and the new awareness of empirical chemical formulas as targets for synthesis by academic chemists. The dye industry became one of the first instances where directed scientific research lead to new products, and the first where this occurred regularly.



















