
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

2,2,6,6-Tetramethylpiperidine, abbreviated TMP, HTMP, or TMPH, is an organic compound of the amine class. In appearance, it is a colorless liquid and has a "fishy", amine-like odor. This amine is used in chemistry as a hindered base. Although TMP finds limited use per se, its derivatives are a mainstay of hindered amine light stabilizers.
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon. This chemical reaction is useful in the organic synthesis of organic compounds.

Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.

N,N-Diisopropylethylamine, or Hünig's base, is an organic compound that is a tertiary amine. It is named after the German chemist Siegfried Hünig. It is used in organic chemistry as a non-nucleophilic base. It is commonly abbreviated as DIPEA,DIEA, or i-Pr2NEt.
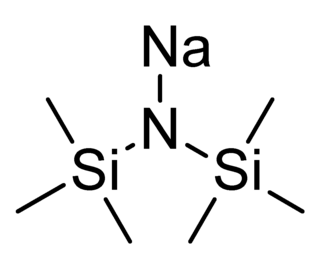
Sodium bis(trimethylsilyl)amide is the organosilicon compound with the formula NaN(Si 3)2. This species, usually called NaHMDS, is a strong base used for deprotonation reactions or base-catalyzed reactions. Its advantages are that it is commercially available as a solid and it is soluble not only in ethers, such as THF or diethyl ether, but also in aromatic solvents, like benzene and toluene by virtue of the lipophilic TMS groups.
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.

2,6-Dimethylpiperidines are chemical compounds with the formula C5H8(CH3)2NH. Three stereoisomers exist: the achiral (R,S)-isomer and the chiral (R,R)/(S,S) enantiomeric pair. Dimethylpiperidines are derivatives of the heterocycle piperidine, wherein two hydrogen atoms are replaced by methyl groups.

Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(Si(CH3)3)2. It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.

Hindered amine light stabilizers (HALS) are chemical compounds containing an amine functional group that are used as stabilizers in plastics and polymers. These compounds are typically derivatives of tetramethylpiperidine and are primarily used to protect the polymers from the effects of photo-oxidation; as opposed to other forms of polymer degradation such as ozonolysis. They are also increasingly being used as thermal stabilizers, particularly for low and moderate level of heat, however during the high temperature processing of polymers they remain less effective than traditional phenolic antioxidants.
Pempidine is a ganglion-blocking drug, first reported in 1958 by two research groups working independently, and introduced as an oral treatment for hypertension.

Borane dimethylsulfide (BMS) is a chemical compound with the chemical formula BH3·S(CH3)2. It is an adduct between borane molecule and dimethyl sulfide molecule. It is a complexed borane reagent that is used for hydroborations and reductions. The advantages of BMS over other borane reagents, such as borane-tetrahydrofuran, are its increased stability and higher solubility. BMS is commercially available at much higher concentrations than its tetrahydrofuran counterpart and does not require sodium borohydride as a stabilizer, which could result in undesired side reactions. In contrast, BH3·THF requires sodium borohydride to inhibit reduction of THF to tributyl borate. BMS is soluble in most aprotic solvents.

(2,2,6,6-Tetramethylpiperidin-1-yl)oxyl or (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl, commonly known as TEMPO, is a chemical compound with the formula (CH2)3(CMe2)2NO. This heterocyclic compound is a red-orange, sublimable solid. As a stable aminoxyl radical, it has applications in chemistry and biochemistry. TEMPO is used as a radical marker, as a structural probe for biological systems in conjunction with electron spin resonance spectroscopy, as a reagent in organic synthesis, and as a mediator in controlled radical polymerization.

4-Hydroxy-TEMPO or TEMPOL, formally 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl, is a heterocyclic compound. Like the related TEMPO, it is used as a catalyst and chemical oxidant by virtue of being a stable aminoxyl radical. Its major appeal over TEMPO is that it is less expensive, being produced from triacetone amine, which is itself made via the condensation of acetone and ammonia. This makes it economically viable on an industrial scale.
An organic radical battery (ORB) is a type of battery first developed in 2005. As of 2011, this type of battery was generally not available for the consumer, although their development at that time was considered to be approaching practical use. ORBs are potentially more environmentally friendly than conventional metal-based batteries, because they use organic radical polymers to provide electrical power instead of metals. ORBs are considered to be a high-power alternative to the Li-ion battery. Functional prototypes of the battery have been researched and developed by different research groups and corporations including the Japanese corporation NEC.
Di-tert-butyl-iminodicarboxylate is an organic compound that can be described with the formula [(CH3)3COC(O)]2NH. It is a white solid that is soluble in organic solvents. The compound is used as a reagent for the preparation of primary amines from alkyl halides. It was popularized as an alternative to the Gabriel synthesis for the same conversion. Amines can also be prepared from alcohols by dehydration using the Mitsunobu reaction.
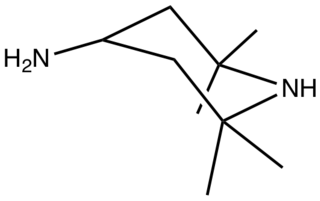
4-Amino-2,2,6,6-tetramethyl-4-piperidine is an organic compound with the formula H2NCH(CH2CMe2)2NH (where Me = CH3). Classified as a diamine, it is a colorless oily liquid.

Bobbitt's salt is an oxoammonium compound derived from 4-acetamido-2,2,6,6-tetramethylpiperidine. It contains the tetrafluoroborate anion and is named after the American chemist James M. Bobbitt.

1-Hydroxy-2,2,6,6-tetramethylpiperidine is the organic compound with the formula C5H6Me4NOH (Me = CH3). A white solid, it is classified as a hydroxylamine. The compound has attracted interest as the reduced derivative of the popular radical 2,2,6,6-tetramethylpiperidin-1-yl)oxyl ("TEMPO"). It is a mild base.
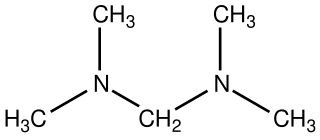
Bis(dimethylamino)methane is the organic compound with the formula [(CH3)2N]2CH2. It is classified as an aminal as well as a ditertiary amine, in fact the simplest. It is a colorless liquid that is widely available. It is prepared by the reaction of dimethylamine and formaldehyde:


















