
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

Tin is a chemical element with the symbol Sn and atomic number 50. A silvery-coloured metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, the so-called "tin cry" can be heard as a result of twinning in tin crystals; this trait is shared by indium, cadmium, zinc, and mercury in its solid state.

Biofouling or biological fouling is the accumulation of microorganisms, plants, algae, or small animals where it is not wanted on surfaces such as ship and submarine hulls, devices such as water inlets, pipework, grates, ponds, and rivers that cause degradation to the primary purpose of that item. Such accumulation is referred to as epibiosis when the host surface is another organism and the relationship is not parasitic. Since biofouling can occur almost anywhere water is present, biofouling poses risks to a wide variety of objects such as boat hulls and equipment, medical devices and membranes, as well as to entire industries, such as paper manufacturing, food processing, underwater construction, and desalination plants.

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula [(CH3)2C(CN)]2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rubber and as a radical initiator.

Tetrabutyltin is the organotin compound with the molecular formula Sn(CH2CH2CH2CH3)4 or SnBu4, where Bu is butyl −CH2CH2CH2CH3. Sometimes abbreviated TTBT, it is a colorless, lipophilic oil.

Tributyltin (TBT) is an umbrella term for a class of organotin compounds which contain the (C4H9)3Sn group, with a prominent example being tributyltin oxide. For 40 years TBT was used as a biocide in anti-fouling paint, commonly known as bottom paint, applied to the hulls of oceangoing vessels. Bottom paint improves ship performance and durability as it reduces the rate of biofouling, the growth of organisms on the ship's hull. The TBT slowly leaches out into the marine environment where it is highly toxic toward nontarget organisms. TBT toxicity can lead to biomagnification or bioaccumulation within such nontarget organisms like invertebrates, vertebrates, and a variety of mammals. TBT is also an obesogen. After it led to collapse of local populations of organisms, TBT was banned.
Imposex is a disorder in sea snails caused by the toxic effects of certain marine pollutants. These pollutants cause female sea snails to develop male sex organs such as a penis and a vas deferens.

Picolinic acid is an organic compound with the formula C5H4N(CO2H). It is a derivative of pyridine with a carboxylic acid (COOH) substituent at the 2-position. It is an isomer of nicotinic acid and isonicotinic acid, which have the carboxyl side chain at the 3- and 4-position, respectively. It is a white solid that is soluble in water.
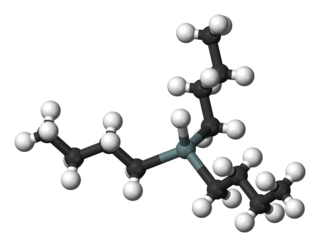
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis.

Triphenyltin chloride is an organotin compound with formula Sn(C6H5)3Cl. It is a colourless solid that dissolves in organic solvents. It slowly reacts with water. The main use for this compound is as a fungicide and antifoulant. Triphenyl tin chloride is used as a chemosterilant. Triphenyl tins used as an antifeedants against potato cutworm.

Dibutyltin oxide, or dibutyloxotin, is an organotin compound with the chemical formula (C4H9)2SnO. It is a colorless solid that, when pure, is insoluble in organic solvents. It is used as a reagent and a catalyst.

Stannole is an organotin compound with the formula (CH)4SnH2. It is classified as a metallole, i.e. an unsaturated five-membered ring containing a heteroatom. It is a structural analog of cyclopentadiene, with tin replacing the saturated carbon atom. Substituted derivatives, which have been synthesized, are also called stannoles.
Triphenyltin compounds are organotin compounds with the general formula (C6H5)3SnX. They contain the triphenyltin group, (C6H5)3Sn, or Ph3Sn, which consists of an atom of tin bonded to three phenyl groups. Examples of triphenyltins include:
Tin poisoning refers to the toxic effects of tin and its compounds. Cases of poisoning from tin metal, its oxides, and its salts are "almost unknown"; on the other hand, certain organotin compounds are almost as toxic as cyanide.
Alwyn George Davies FRS is a British chemist, emeritus professor, and Fellow of University College London.

Dibutyltin dilaurate is an organotin compound with the formula (CH310CO2)2Sn( 3CH3)2. It is a colorless viscous and oily liquid. It is used as a catalyst.

Stannoxane is a functional group in organotin chemistry with the connectivity SnIV−O−SnIV. Aside from the oxide group, usually 3 or 4 other substituents are attached to tin. In aqueous or aquatic environments, most organotin compounds contain this group.
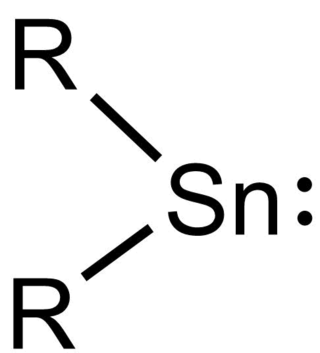
Stannylenes (R2Sn:) are a class of organotin(II) compounds that are analogues of carbene. Unlike carbene, which usually has a triplet ground state, stannylenes have a singlet ground state since valence orbitals of tin (Sn) have less tendency to form hybrid orbitals and thus the electrons in 5s orbital are still paired up. Free stannylenes are stabilized by steric protection. Adducts with Lewis bases are also known.

Tributyltin chloride is an organotin compound with the formula (C4H9)3SnCl. It is a colorless liquid that is soluble in organic solvents.




















