Nitrogen fixation is a chemical process by which molecular nitrogen (N
2), which has a strong triple covalent bond, is converted into ammonia (NH
3) or related nitrogenous compounds, typically in soil or aquatic systems but also in industry. The nitrogen in air is molecular dinitrogen, a relatively nonreactive molecule that is metabolically useless to all but a few microorganisms. Biological nitrogen fixation or diazotrophy is an important microbe-mediated process that converts dinitrogen (N2) gas to ammonia (NH3) using the nitrogenase protein complex (Nif).

Cyanobacteria, also called Cyanobacteriota or Cyanophyta, are a phylum of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" refers to their cyan color, which forms the basis of cyanobacteria's informal common name, blue-green algae, although as prokaryotes they are not scientifically classified as algae. They appear to have originated in a freshwater or terrestrial environment.

Trichodesmium, also called sea sawdust, is a genus of filamentous cyanobacteria. They are found in nutrient poor tropical and subtropical ocean waters. Trichodesmium is a diazotroph; that is, it fixes atmospheric nitrogen into ammonium, a nutrient used by other organisms. Trichodesmium is thought to fix nitrogen on such a scale that it accounts for almost half of the nitrogen fixation in marine systems globally. Trichodesmium is the only known diazotroph able to fix nitrogen in daylight under aerobic conditions without the use of heterocysts.
Diazotrophs are bacteria and archaea that fix atmospheric nitrogen(N2) in the atmosphere into bioavailable forms such as ammonia.

Oscillatoria is a genus of sugar making microscopic creatures.
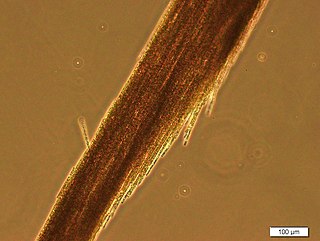
Aphanizomenon flos-aquae is a brackish and freshwater species of cyanobacteria of the genus Aphanizomenon found around the world, including the Baltic Sea and the Great Lakes.

Cyanophages are viruses that infect cyanobacteria, also known as Cyanophyta or blue-green algae. Cyanobacteria are a phylum of bacteria that obtain their energy through the process of photosynthesis. Although cyanobacteria metabolize photoautotrophically like eukaryotic plants, they have prokaryotic cell structure. Cyanophages can be found in both freshwater and marine environments. Marine and freshwater cyanophages have icosahedral heads, which contain double-stranded DNA, attached to a tail by connector proteins. The size of the head and tail vary among species of cyanophages. Cyanophages infect a wide range of cyanobacteria and are key regulators of the cyanobacterial populations in aquatic environments, and may aid in the prevention of cyanobacterial blooms in freshwater and marine ecosystems. These blooms can pose a danger to humans and other animals, particularly in eutrophic freshwater lakes. Infection by these viruses is highly prevalent in cells belonging to Synechococcus spp. in marine environments, where up to 5% of cells belonging to marine cyanobacterial cells have been reported to contain mature phage particles.

Aphanizomenon is a genus of cyanobacteria that inhabits freshwater lakes and can cause dense blooms. They are unicellular organisms that consolidate into linear (non-branching) chains called trichomes. Parallel trichomes can then further unite into aggregates called rafts. Cyanobacteria such as Aphanizomenon are known for using photosynthesis to create energy and therefore use sunlight as their energy source. Aphanizomenon bacteria also play a big role in the Nitrogen cycle since they can perform nitrogen fixation. Studies on the species Aphanizomenon flos-aquae have shown that it can regulate buoyancy through light-induced changes in turgor pressure. It is also able to move by means of gliding, though the specific mechanism by which this is possible is not yet known.
Cyanobionts are cyanobacteria that live in symbiosis with a wide range of organisms such as terrestrial or aquatic plants; as well as, algal and fungal species. They can reside within extracellular or intracellular structures of the host. In order for a cyanobacterium to successfully form a symbiotic relationship, it must be able to exchange signals with the host, overcome defense mounted by the host, be capable of hormogonia formation, chemotaxis, heterocyst formation, as well as possess adequate resilience to reside in host tissue which may present extreme conditions, such as low oxygen levels, and/or acidic mucilage. The most well-known plant-associated cyanobionts belong to the genus Nostoc. With the ability to differentiate into several cell types that have various functions, members of the genus Nostoc have the morphological plasticity, flexibility and adaptability to adjust to a wide range of environmental conditions, contributing to its high capacity to form symbiotic relationships with other organisms. Several cyanobionts involved with fungi and marine organisms also belong to the genera Richelia, Calothrix, Synechocystis, Aphanocapsa and Anabaena, as well as the species Oscillatoria spongeliae. Although there are many documented symbioses between cyanobacteria and marine organisms, little is known about the nature of many of these symbioses. The possibility of discovering more novel symbiotic relationships is apparent from preliminary microscopic observations.
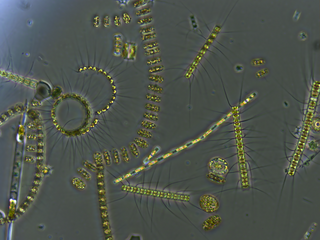
Bacterioplankton refers to the bacterial component of the plankton that drifts in the water column. The name comes from the Ancient Greek word πλανκτος, meaning "wanderer" or "drifter", and bacterium, a Latin term coined in the 19th century by Christian Gottfried Ehrenberg. They are found in both seawater and freshwater.

Planktothrix is a diverse genus of filamentous cyanobacteria observed to amass in algal blooms in water ecosystems across the globe. Like all Oscillatoriales, Planktothrix species have no heterocysts and no akinetes. Planktothrix are unique because they have trichomes and contain gas vacuoles unlike typical planktonic organisms. Previously, some species of the taxon were grouped within the genus Oscillatoria, but recent work has defined Planktothrix as its own genus. A tremendous body of work on Planktothrix ecology and physiology has been done by Anthony E. Walsby, and the 55.6 kb microcystin synthetase gene which gives these organisms the ability to synthesize toxins has been sequenced. P. agardhii is an example of a type species of the genus. P. agardhii and P. rubescens are commonly observed in lakes of the Northern Hemisphere where they are known producers of potent hepatotoxins called microcystins.
CandidatusAtelocyanobacterium thalassa, also referred to as UCYN-A, is a diazotrophic species of cyanobacteria commonly found in measurable quantities throughout the world's oceans and some seas. Members of A. thalassa are spheroid in shape and are 1-2 μm in diameter, and provide nitrogen to ocean regions by fixing non biologically available atmospheric nitrogen into biologically available ammonium that other marine microorganisms can use.
Raphidiopsis raciborskii is a freshwater cyanobacterium.

Cyanothece is a genus of unicellular, diazotrophic, oxygenic photosynthesizing cyanobacteria.
Trichodesmium erythraeum is a marine cyanobacteria species characterized by its prolific diazotrophic capabilities. They play a dominant role in the ocean ecosystem, supplying a steady and significant source of new, biologically available nitrogen and cycling phosphorus. By nature of its filamentous morphology, T. erythraeum is also known to congregate into large, long colonies, sizeable enough to be seen as sawdust-like particles to the naked eye and pigmented marine regions in satellite images, typically found in oligotrophic tropical and subtropical waters. These blooms are responsible for the famous coloration of the Red Sea.

Crocosphaera watsonii is an isolate of a species of unicellular diazotrophic marine cyanobacteria which represent less than 0.1% of the marine microbial population. They thrive in offshore, open-ocean oligotrophic regions where the waters are warmer than 24 degrees Celsius. Crocosphaera watsonii cell density can exceed 1,000 cells per milliliter within the euphotic zone; however, their growth may be limited by the concentration of phosphorus. Crocosphaera watsonii are able to contribute to the oceanic carbon and nitrogen budgets in tropical oceans due to their size, abundance, and rapid growth rate. Crocosphaera watsonii are unicellular nitrogen fixers that fix atmospheric nitrogen to ammonia during the night and contribute to new nitrogen in the oceans. They are a major source of nitrogen to open-ocean systems. Nitrogen fixation is important in the oceans as it not only allows phytoplankton to continue growing when nitrogen and ammonium are in very low supply but it also replenishes other forms of nitrogen, thus fertilizing the ocean and allowing more phytoplankton growth.

Oscillatoria brevis is a species of the genus Oscillatoria first identified in 1892. It is a blue-green filamentous cyanobacterium, which can be found in brackish and fresh waterways. O. brevis can also be isolated from soil.
Richelia is a genus of nitrogen-fixing, filamentous, heterocystous and cyanobacteria. It contains the single species Richelia intracellularis. They exist as both free-living organisms as well as symbionts within potentially up to 13 diatoms distributed throughout the global ocean. As a symbiont, Richelia can associate epiphytically and as endosymbionts within the periplasmic space between the cell membrane and cell wall of diatoms.
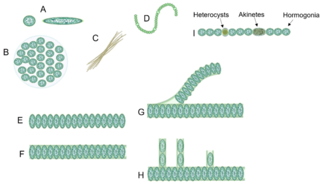
Cyanobacterial morphology refers to the form or shape of cyanobacteria. Cyanobacteria are a large and diverse phylum of bacteria defined by their unique combination of pigments and their ability to perform oxygenic photosynthesis.
Margaret Ruth Mulholland is professor at Old Dominion University known for her work on nutrients in marine and estuarine environments.










