Deubiquitinating enzymes (DUBs), also known as deubiquitinating peptidases, deubiquitinating isopeptidases, deubiquitinases, ubiquitin proteases, ubiquitin hydrolases, ubiquitin isopeptidases, are a large group of proteases that cleave ubiquitin from proteins. Ubiquitin is attached to proteins in order to regulate the degradation of proteins via the proteasome and lysosome; coordinate the cellular localisation of proteins; activate and inactivate proteins; and modulate protein-protein interactions. DUBs can reverse these effects by cleaving the peptide or isopeptide bond between ubiquitin and its substrate protein. In humans there are nearly 100 DUB genes, which can be classified into two main classes: cysteine proteases and metalloproteases. The cysteine proteases comprise ubiquitin-specific proteases (USPs), ubiquitin C-terminal hydrolases (UCHs), Machado-Josephin domain proteases (MJDs) and ovarian tumour proteases (OTU). The metalloprotease group contains only the Jab1/Mov34/Mpr1 Pad1 N-terminal+ (MPN+) (JAMM) domain proteases.

Ubiquitin specific peptidase 9, Y-linked , also known as USP9Y, is an enzyme which in humans is encoded by the USP9Y gene. It is required for sperm production. This enzyme is a member of the peptidase C19 family and is similar to ubiquitin-specific proteases, which cleave the ubiquitin moiety from ubiquitin-fused precursors and ubiquitinylated proteins.

Ras GTPase-activating protein-binding protein 1 is an enzyme that in humans is encoded by the G3BP1 gene.

Ubiquitin-specific-processing protease 7 (USP7), also known as ubiquitin carboxyl-terminal hydrolase 7 or herpesvirus-associated ubiquitin-specific protease (HAUSP), is an enzyme that in humans is encoded by the USP7 gene.
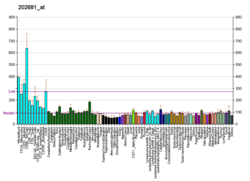
Ubiquitin carboxyl-terminal hydrolase 8 is an enzyme that in humans is encoded by the USP8 gene.

Ubiquitin carboxyl-terminal hydrolase 6 (USB6), also termed TRE17 and Tre-2, is a deubiquitinating enzyme that in humans is encoded by the hominid USP6 gene located at band 13.2 on the short arm of chromosome 17. Deubiquitinating enzymes (DUBs) are enzymes that act within cells to remove ubiquitins from various functionally important proteins. Ubiquitin enzymes add ubiquitin to these proteins and thereby regulate their cellular location, alter their activity, and/or promote their degradation. By deubiquitinating these proteins, DUBs counter the effects of the ubiquinating enzymes and contribute to regulating the actions of the targeted proteins. In normal adult tissues, USP6 is highly expressed in testicle tissue, modestly expressed in ovarian tissue, and absent or minimally expressed in other tissues. It is also highly expressed in fetal brain tissue. The specific functions of USP6 are poorly defined primarily because its presence is restricted to primates: there are no available animal models to determine the effects of its deletion, although some studies suggest that UPSP6 contributes to normal brain development. In all events, USP6 has gained wide interest because of its abnormally increased expression by the neoplastic cells in various tumors derived from mesenchymal tissue.

Ubiquitin specific peptidase 10, also known as USP10, is an enzyme which in humans is encoded by the USP10 gene.

Ubiquitin conjugation factor E4 B is a protein that in humans is encoded by the UBE4B gene.

Ubiquitin carboxyl-terminal hydrolase 16 is an enzyme that in humans is encoded by the USP16 gene.

Ubiquitin carboxyl-terminal hydrolase or Ubiquitin specific protease 11 is an enzyme that in humans is encoded by the USP11 gene. USP11 belongs to the Ubiquitin specific proteases family (USPs) which is a sub-family of the Deubiquitinating enzymes (DUBs).USPs are multiple domain proteases and belong to the C19 cysteine proteases sub‒family. Depending on their domain architecture and position there is different homology between the various members. Generally the largest domain is the catalytic domain which harbours the three residue catalytic triad that is included inside conserved motifs. The catalytic domain also contains sequences that are not related with the catalysis function and their role is mostly not clearly understood at present, the length of these sequences varies for each USP and therefore the length of the whole catalytic domain can range from approximately 295 to 850 amino acids. Particular sequences inside the catalytic domain or at the N‒terminus of some USPs have been characterised as UBL and DUSP domains respectively. In some cases, regarding the UBL domains, it has been reported to have a catalysis enhancing function as in the case of USP7. In addition, a so‒called DU domain module is the combination of a DUSP domain followed by a UBL domain separated by a linker and is found in USP11 as well as in USP15 and USP4.

Ubiquitin-specific protease 36 is an enzyme that in humans is encoded by the USP36 gene.

Ubiquitin carboxyl-terminal hydrolase 1 is an enzyme that in humans is encoded by the USP1 gene.

Ubiquitin carboxyl-terminal hydrolase 15 is an enzyme that in humans is encoded by the USP15 gene.

Ubiquitin carboxyl-terminal hydrolase 33 is an enzyme that in humans is encoded by the USP33 gene.

Ubiquitin carboxyl-terminal hydrolase 48 is an enzyme that in humans is encoded by the USP48 gene.

Ubiquitin carboxyl-terminal hydrolase 2 is an enzyme that in humans is encoded by the USP2 gene.

Ubiquitin carboxyl-terminal hydrolase 13 is an enzyme that in humans is encoded by the USP13 gene.

Ubiquitin-specific protease 14 is an enzyme that in humans is encoded by the USP14 gene.

Ubiquitin carboxyl-terminal hydrolase 20 is an enzyme that in humans is encoded by the USP20 gene.

The ubiquitin carboxyl-terminal hydrolase 27, also known as deubiquitinating enzyme 27, ubiquitin thioesterase 27 and USP27X, is a deubiquitinating enzyme which is mainly characterized for cleaving ubiquitin (Ub) from proteins and other molecules. Ubiquitin binds to proteins so as to regulate the degradation of them via the proteasome and lysosome among many other functions.