Contents
| |||
| Names | |||
|---|---|---|---|
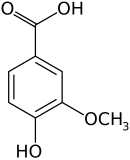
| Preferred IUPAC name 4-Hydroxy-3-methoxybenzoic acid | |||
| Other names 4-Hydroxy-m-anisic acid, Vanillate | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.061 | ||
| KEGG | |||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C8H8O4 | |||
| Molar mass | 168.148 g·mol−1 | ||
| Appearance | White to light yellow powder or crystals | ||
| Melting point | 210 to 213 °C (410 to 415 °F; 483 to 486 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds | Vanillin, vanillyl alcohol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Vanillic acid (4-hydroxy-3-methoxybenzoic acid) is a dihydroxybenzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin. It is also an intermediate in the production of vanillin from ferulic acid. [2] [3]