
Catalysis is the increase in rate of a chemical reaction due to an added substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.

A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.

An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for the reactions involving the light (kL) and the heavy (kH) isotopically substituted reactants (isotopologues):

Vinyl alcohol, also called ethenol or ethylenol, is the simplest enol. With the formula CH2CHOH, it is a labile compound that converts to acetaldehyde immediately upon isolation near room temperature. It is not a practical precursor to any compound.
In chemistry, molecularity is the number of molecules that come together to react in an elementary (single-step) reaction and is equal to the sum of stoichiometric coefficients of reactants in the elementary reaction with effective collision and correct orientation. Depending on how many molecules come together, a reaction can be unimolecular, bimolecular or even trimolecular.

The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation (Δ = −327 kcal/mol.

Robert Goulston Gilbert is a polymer chemist whose most significant contributions have been in the field of emulsion polymerisation. In 1970, he gained his PhD from the Australian National University, and worked at the University of Sydney from then until 2006. In 1982, he was elected a fellow of the Royal Australian Chemical Institute; in 1994, he was elected a fellow of the Australian Academy of Science. In 1992, he was appointed full professor, and in 1999 he started the Key Centre for Polymer Colloids, funded by the Australian Research Council, the University and industry. He has served in leadership roles in the International Union of Pure and Applied Chemistry (IUPAC), the world ‘governing body’ of chemistry. He was founding chair (1987–98) of the IUPAC Working Party on the Modelling of Kinetics Processes of Polymerisation, of which he remains a member, and is a member of the IUPAC scientific task groups on starch molecular weight measurements, and terminology. He was vice-president (1996–97) and president (1998–2001) of the IUPAC Macromolecular Division, and secretary of the International Polymer Colloids Group (1997–2001). As of 2007, he is Research Professor at the Centre of Nutrition and Food Science, University of Queensland, where his research program concentrates on the relations between starch structure and nutrition.

Frank Henry Westheimer was an American chemist. He taught at the University of Chicago from 1936 to 1954, and at Harvard University from 1953 to 1983, becoming the Morris Loeb Professor of Chemistry in 1960, and Professor Emeritus in 1983. The Westheimer medal was established in his honor in 2002.
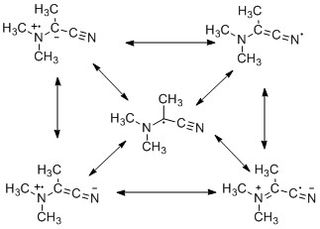
The captodative effect is the stabilization of radicals by a synergistic effect of an electron-withdrawing substituent and an electron-donating substituent. The name originates as the electron-withdrawing group (EWG) is sometimes called the "captor" group, whilst the electron-donating group (EDG) is the "dative" substituent. Olefins with this substituent pattern are sometime described as captodative. Radical reactions play an integral role in several chemical reactions and are also important to the field of polymer science.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.
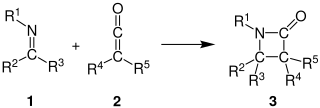
The Staudinger synthesis, also called the Staudinger ketene-imine cycloaddition, is a chemical synthesis in which an imine 1 reacts with a ketene 2 through a non-photochemical 2+2 cycloaddition to produce a β-lactam3. The reaction carries particular importance in the synthesis of β-lactam antibiotics. The Staudinger synthesis should not be confused with the Staudinger reaction, a phosphine or phosphite reaction used to reduce azides to amines.

Reaction Design is a San Diego-based developer of combustion simulation software used by engineers to design cleaner burning and fuel-efficient combustors and engines, found in everything from automobiles to turbines for power generation and aircraft propulsion to large diesel engines that use pistons the size of rooms to propel ships locomotives. The technology is also used to model spray vaporization in electronic materials processing applications and predict mixing reactions in chemical plants. Ansys, a leader in engineering simulation software, acquired Reaction Design in January 2014.
Sharon Hammes-Schiffer is a physical chemist who has contributed to theoretical and computational chemistry. She is currently a Sterling Professor of Chemistry at Yale University. She has served as senior editor and deputy editor of the Journal of Physical Chemistry and advisory editor for Theoretical Chemistry Accounts. As of 1 January 2015 she is editor-in-chief of Chemical Reviews.
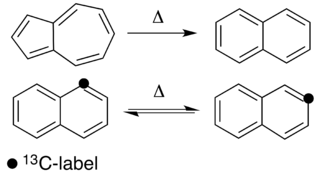
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs (automerization). The general reaction schemes of the two types are illustrated in Figure 1.
Karen Ila Goldberg is an American chemist, currently the Vagelos Professor of Energy Research at University of Pennsylvania. Goldberg is most known for her work in inorganic and organometallic chemistry. Her most recent research focuses on catalysis, particularly on developing catalysts for oxidation, as well as the synthesis and activation of molecular oxygen. In 2018, Goldberg was elected to the National Academy of Sciences.
Charles Bradley Moore is an American chemist and research administrator. His research focused on the application of lasers to understand the behavior and reaction dynamics of energized molecules and energy transfer between molecules. He is currently professor emeritus of chemistry at the University of California, Berkeley, where he had a long career as a faculty member actively engaged in research (1963–2000). While at UC Berkeley, Moore also served in several administrative roles, including chair of the department of chemistry (1982–1986), dean of the college of chemistry (1988–1994), and director of the chemical sciences division at the Lawrence Berkeley National Laboratory (1998–2000). He was vice president for research at Ohio State University (2000–2003) and held the same position at Northwestern University (2004–2008). He is also professor emeritus at Northwestern. Moore is a member of the National Academy of Sciences.
Alexis Tarassov Bell is an American chemical engineer. He is currently the Dow professor of Sustainable Chemistry in the Department of Chemical and Biomolecular Engineering in UC Berkeley's college of chemistry. He is also the Faculty Senior Scientist at Lawrence Berkeley National Laboratory. He is known for his work with heterogenous catalysts and characterizing the mechanisms of these reactions on a quantum level.












