
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel (aggregate) together. Cement mixed with fine aggregate produces mortar for masonry, or with sand and gravel, produces concrete. Concrete is the most widely used material in existence and is behind only water as the planet's most-consumed resource.
Cement chemist notation (CCN) was developed to simplify the formulas cement chemists use on a daily basis. It is a shorthand way of writing the chemical formula of oxides of calcium, silicon, and various metals.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.
In chemistry, an aluminate is a compound containing an oxyanion of aluminium, such as sodium aluminate. In the naming of inorganic compounds, it is a suffix that indicates a polyatomic anion with a central aluminium atom.

Brownmillerite is a rare oxide mineral with chemical formula Ca2(Al,Fe)2O5. It is named for Lorrin Thomas Brownmiller (1902–1990), chief chemist of the Alpha Portland Cement Company, Easton, Pennsylvania.

Bentorite is a mineral with the chemical formula Ca6(Cr,Al)2(SO4)3(OH)12·26H2O. It is colored violet to light violet. Its crystals are hexagonal to dihexagonal dipyramidal. It is transparent and has vitreous luster. It has perfect cleavage. It is not radioactive. Bentorite is rated 2 on the Mohs scale.

Ettringite is a hydrous calcium aluminium sulfate mineral with formula: Ca6Al2(SO4)3(OH)12·26H2O. It is a colorless to yellow mineral crystallizing in the trigonal system. The prismatic crystals are typically colorless, turning white on partial dehydration. It is part of the ettringite-group which includes other sulfates such as thaumasite and bentorite.
Alite is an impure form of tricalcium silicate, Ca3SiO5, sometimes formulated as 3CaO·SiO2, typically with 3-4% of substituent oxides. It is the major, and characteristic, phase in Portland cement. The name was given by Törnebohm in 1897 to a crystal identified in microscopic investigation of Portland cement. Hatrurite is the name of a mineral that is substituted C3S.
Belite is an industrial mineral important in Portland cement manufacture. Its main constituent is dicalcium silicate, Ca2SiO4, sometimes formulated as 2 CaO · SiO2 (C2S in cement chemist notation).

Cement clinker is a solid material produced in the manufacture of portland cement as an intermediary product. Clinker occurs as lumps or nodules, usually 3 millimetres (0.12 in) to 25 millimetres (0.98 in) in diameter. It is produced by sintering limestone and aluminosilicate materials such as clay during the cement kiln stage.
Tricalcium aluminate Ca3Al2O6, often formulated as 3CaO·Al2O3 to highlight the proportions of the oxides from which it is made, is the most basic of the calcium aluminates. It does not occur in nature, but is an important mineral phase in Portland cement.

Calcium aluminates are a range of materials obtained by heating calcium oxide and aluminium oxide together at high temperatures. They are encountered in the manufacture of refractories and cements.
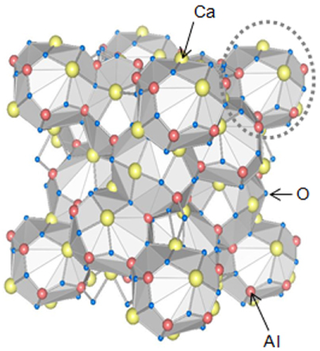
Dodecacalcium hepta-aluminate (12CaO·7Al2O3, Ca12Al14O33 or C12A7) is an inorganic solid that occurs rarely in nature as the mineral mayenite. It is an important phase in calcium aluminate cements and is an intermediate in the manufacture of Portland cement. Its composition and properties have been the subject of much debate, because of variations in composition that can arise during its high-temperature formation.

Calcium aluminate cements are cements consisting predominantly of hydraulic calcium aluminates. Alternative names are "aluminous cement", "high-alumina cement", and "Ciment fondu" in French. They are used in a number of small-scale, specialized applications.

Calcium aluminoferrite is a dark brown crystalline phase commonly found in cements. In the cement industry it is termed tetra-calcium aluminoferrite or ferrite. In cement chemist notation (CCN), it is abbreviated as C
4AF meaning 4CaO·Al
2O
3·Fe
2O
3 in the oxide notation. It also exists in nature as the rare mineral brownmillerite.
An AFm phase is an "alumina, ferric oxide, monosubstituted" phase, or aluminate ferrite monosubstituted, or Al2O3, Fe2O3 mono, in cement chemist notation (CCN). AFm phases are important hydration products in the hydration of Portland cements and hydraulic cements.

Chlormayenite (after Mayen, Germany), Ca12Al14O32[☐4Cl2], is a rare calcium aluminium oxide mineral of cubic symmetry.
The Hatrurim Formation or Mottled Zone is a geologic formation with outcrops all around the Dead Sea Basin: in the Negev Desert in Israel, in the Judaean Desert on the West Bank, and in western Jordan. It includes late Cretaceous to Eocene aged impure limestone along with coal bearing chalk and marl. The rocks have been subjected to pyrometamorphism resulting from combustion of contained or underlying coal or hydrocarbon deposits. The formation is named for exposures in the Hatrurim Basin which lies west of the Dead Sea.
Cement hydration and strength development mainly depend on two silicate phases: tricalcium silicate (C3S) (alite), and dicalcium silicate (C2S) (belite). Upon hydration, the main reaction products are calcium silicate hydrates (C-S-H) and calcium hydroxide Ca(OH)2, written as CH in the cement chemist notation. C-S-H is the phase playing the role of the glue in the cement hardened paste and responsible of its cohesion. Cement also contains two aluminate phases: C3A and C4AF, respectively the tricalcium aluminate and the tetracalcium aluminoferrite. C3A hydration products are AFm, calcium aluminoferrite monosulfate, and ettringite, a calcium aluminoferrite trisulfate (AFt). C4AF hydrates as hydrogarnet and ferrous ettringite.
AFt Phases refer to the calcium Aluminate Ferrite trisubstituted, or calcium aluminate trisubstituted, phases present in hydrated cement paste (HCP) in concrete.








