
Copaene, or more precisely, α-copaene, is the common chemical name of an oily liquid hydrocarbon that is found in a number of essential oil-producing plants. The name is derived from that of the resin-producing tropical copaiba tree, Copaifera langsdorffii, from which the compound was first isolated in 1914. Its structure, including the chirality, was determined in 1963. The double-bond isomer with an exocyclic-methylene group, β-copaene, was first reported in 1967.

Cupferron is jargon for the ammonium salt of the conjugate base derived from N-nitroso-N-phenylhydroxylamine. It once was a common reagent for the complexation of metal ions, being of interest in the area of qualitative inorganic analysis. Its formula is NH4[C6H5N(O)NO]. The anion binds to metal cations through the two oxygen atoms, forming five-membered chelate rings.

Ditellurium bromide is the inorganic compound with the formula Te2Br. It is one of the few stable lower bromides of tellurium. Unlike sulfur and selenium, tellurium forms families of polymeric subhalides where the chalcogen/halide ratio is less than 2.

Tritellurium dichloride is the inorganic compound with the formula Te3Cl2. It is one of the more stable lower chlorides of tellurium.
Selenium hexasulfide is a chemical compound with formula Se2S6. Its molecular structure consists of a ring of two selenium and six sulfur atoms, analogous to the S8 allotrope of sulfur (cyclooctasulfur) and other selenium sulfides with formula SenS8−n.

Methanesulfonic anhydride is the acid anhydride of methanesulfonic acid. Like methanesulfonyl chloride, it may be used to generate mesylates (methanesulfonyl esters). It is commercially available but may also be prepared by the reaction of phosphorus pentoxide with methanesulfonic acid at 80 °C. It can easily be purified by distillation under vacuum (distillation of a solid) or by recrystallized from tert-butylmethylether/toluene.
Silver selenite is an inorganic compound of formula Ag2SeO3.
Iron phosphide is a chemical compound of iron and phosphorus, with a formula of FeP. Its physical appearance is grey, hexagonal needles.
Tellurium iodide is an inorganic compound with the formula TeI. Two forms are known. Their structures differ from the other monohalides of tellurium. There are three subiodides of tellurium, α-TeI, β-TeI, and Te2I, and one tellurium tetraiodide.
Rotational correlation time is the average time it takes for a molecule to rotate one radian. In solution, rotational correlation times are in the order of picoseconds. For example, the 1.7 ps for water, and 100 ps for a pyrroline nitroxyl radical in a DMSO-water mixture. Rotational correlation times are employed in the measurement of microviscosity and in protein characterization.
Arne Haaland is a Norwegian chemist.

Xylobolus frustulatus, commonly known as the ceramic fungus or ceramic parchment, is an inedible species of crust fungus in the Stereaceae family. The fruit body forms small, hard, flat crust-like aggregations that resemble broken pieces of ceramic tile. These pieces are initially whitish before turning yellow-brown to gray-brown in age. The spore-bearing cells cover the upper surfaces of the fruit body. A saprobic species, it grows on well-decayed oak wood in Asia, northern Europe, and North America.
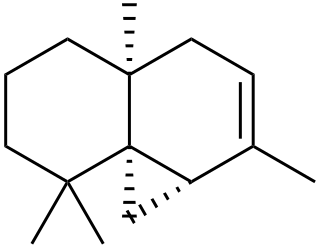
Thujopsene is a natural chemical compound, classified as a sesquiterpene, with the molecular formula C15H24.

Silver sulfite is the chemical compound with the formula Ag2SO3. This unstable silver compound when heated and/or in light it decomposes to silver dithionate and silver sulfate.
Streptomyces armeniacus is a bacterium species from the genus Streptomyces which has isolated from soil. Streptomyces armeniacus produces streptopyrrole.

Trithiapentalene is an organic bicyclic molecule containing two sulfur heterocycles. Its 10-π aromatic structure is similar to naphthalene. There has been a literature dispute about whether the connectivity among the three sulfur atoms is a case of rapid tautomerization between two valence tautomers or a 3-center 4-electron bond.

Methylfluorophosphonylcholine (MFPCh) is an extremely toxic chemical compound related to the G-series nerve agents. It is an extremely potent acetylcholinesterase inhibitor which is around 100 times more potent than sarin at inhibiting acetylcholinesterase in vitro, and around 10 times more potent in vivo, depending on route of administration and animal species tested. MFPCh is resistant to oxime reactivators, meaning the acetylcholinesterase inhibited by MFPCh can't be reactivated by oxime reactivators. MFPCh also acts directly on the acetylcholine receptors. However, despite its high toxicity, methylfluorophosphonylcholine is a relatively unstable compound and degrades rapidly in storage, so it was not deemed suitable to be weaponised for military use.

Karin Aurivillius (1920–1982) was a Swedish chemist and crystallographer at the University of Lund, Sweden. She determined the crystal structures of many mercury compounds.
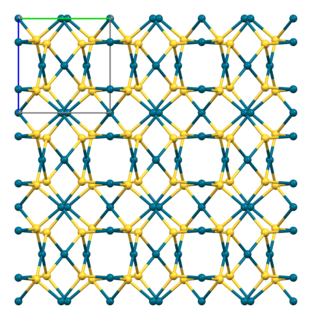
Palladium(II) sulfide is a chemical compound of palladium and sulfur with the chemical formula PdS. Like other palladium and platinum chalcogenides, palladium(II) sulfide has complex structural, electrical and magnetic properties.
Palladium sulfide may refer to:












