
Aminorex is a weight loss (anorectic) stimulant drug. It was withdrawn from the market after it was found to cause pulmonary hypertension. In the U.S., it is an illegal Schedule I drug, meaning it has high abuse potential, no accepted medical use, and a poor safety profile.

Etonitazene, also known as EA-4941 or CS-4640, is a benzimidazole opioid, first reported in 1957, that has been shown to have approximately 1,000 to 1,500 times the potency of morphine in animals.

Rolicyclidine (PCPy) is a dissociative anesthetic that is similar in effects to phencyclidine, but is slightly less potent and has fewer stimulant effects. It instead produces a sedative effect described as being somewhat similar to a barbiturate, but with additional PCP-like dissociative, anaesthetic and hallucinogenic effects. Due to its similarity in effects to PCP, PCPy was placed into the Schedule I list of illegal drugs in the 1970s, although it has never been widely abused and is now little known.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Azaprocin is a drug which is an opioid analgesic with approximately ten times the potency of morphine, and a fast onset and short duration of action. It was discovered in 1963, but has never been marketed.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

BDPC is a potent fully synthetic opioid with a distinctive arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. Initial studies estimated that it was around 10,000 times the strength of morphine in animal models. However, later studies using more modern techniques assigned a value of 504 times the potency of morphine for the more active trans-isomer. This drug was first seized along with three kilograms of acetylfentanyl in an April 25, 2013 police action in Montreal, Canada, and has reportedly continued to be available on the designer drug market internationally. Analogues where the para-bromine is replaced by chlorine or a methyl group retain similar activity, while the meta-hydroxyl derivative demonstrated robust antagonist activity.

(–)-2β-Carbomethoxy-3β-(4-tolyl)tropane is a phenyltropane-based cocaine analogue that has similar properties in vitro to related drugs such as RTI-31.

Biphalin is a dimeric enkephalin endogenous peptide (Tyr-D-Ala-Gly-Phe-NH)2 composed of two tetrapeptides derived from enkephalins, connected 'tail-to-tail' by a hydrazide bridge. The presence of two distinct pharmacophores confers on biphalin a high affinity for both μ and δ opioid receptors (with an EC50 of about 1–5 nM for both μ and δ receptors), therefore it has analgesic activity. Biphalin presents a considerable antinociceptive profile. In fact, when administered intracerebroventricularly in mice, biphalin displays a potency almost 7-fold greater than that of the ultra-potent alkaloid agonist, etorphine and 7000-fold greater than morphine; biphalin and morphine were found to be equipotent after intraperitoneal administration. The extraordinary in vivo potency shown by this compound is coupled with low side-effects, in particular, to produce no dependency in chronic use. For these reasons, several efforts have been carried out in order to obtain more information about structure-activity relationship (SAR). Results clearly indicate that, at least for μ receptor binding, the presence of two pharmacophores is not necessary; Tyr1 is indispensable for analgesic activity, while replacing Phe at the position 4 and 4' with non-aromatic, but lipophilic amino acids does not greatly change the binding properties and in general 4,4' positions are found to be important to design biphalin analogues with increased potency and modified μ/δ selectivity. The hydrazide linker is not fundamental for activity or binding, and it can be conveniently substituted by different conformationally constrained cycloaliphatic diamine linkers.
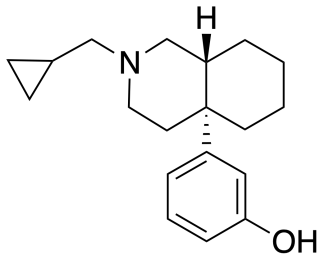
Ciprefadol is an opioid analgesic that is an isoquinoline derivative most closely related to cyclazocine and picenadol, with a number of other related compounds known. Ciprefadol is a mixed agonist–antagonist at μ-opioid receptors and can partly block the effects of morphine at low doses, though at higher doses it acts more like a full agonist. It is also a potent κ-opioid agonist, unlike the corresponding N-methyl and N-phenethyl derivatives which are reasonably μ-selective agonists.

U-47700, also known as U4, pink heroin, pinky, and pink, is an opioid analgesic drug developed by a team at Upjohn in the 1970s which has around 7.5 times the potency of morphine in animal models.

The ProTide technology is a prodrug approach used in molecular biology and drug design. It is designed to deliver nucleotide analogues into the cell. This technology was invented by Professor Chris McGuigan from the School of Pharmacy and Pharmaceutical Sciences at Cardiff University in the early 1990s. ProTides form a critical part of antiviral drugs such as sofosbuvir, tenofovir alafenamide, and remdesivir.
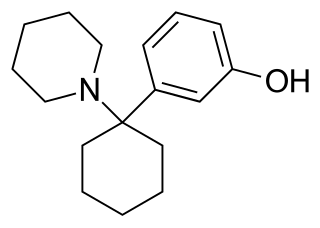
3-Hydroxyphencyclidine (3-HO-PCP) is a dissociative of the arylcyclohexylamine class related to phencyclidine (PCP) that has been sold online as a designer drug.
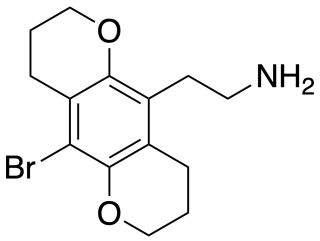
2C-B-BUTTERFLY is a conformationally-restricted derivative of the phenethylamine hallucinogen 2C-B, which was discovered in 1999 by Michael S. Whiteside and Aaron Monte. It is a ring-expanded homologue of the better known compound 2C-B-FLY, and has similar properties as an agonist for serotonin receptors, but with more selectivity for 5-HT2C over 5-HT2A.
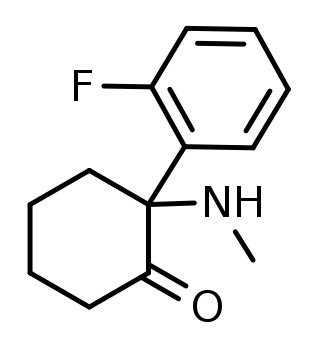
2-Fluorodeschloroketamine is a dissociative anesthetic related to ketamine. Its sale and use as a designer drug has been reported in various countries. It is an analogue of ketamine where the chlorine group has been replaced by fluorine. Due to its recent emergence, the pharmacological specifics of the compound are mostly unclear, but effects are reported to be similar to its parent compound, ketamine.

3-Methyl-PCP is a recreational designer drug with dissociative effects. It is an arylcyclohexylamine derivative, related to drugs such as 3'-MeO-PCP and 3'-Me-PCPy. It was first synthesised in the 1960s, but was only identified on the illicit market in Hungary in September 2020, and was made illegal in Hungary in April 2021.
















