
Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies. It is mainly used as a pain medication, and is also commonly used recreationally, or to make other illicit opioids. There are numerous methods used to administer morphine: oral; sublingual; via inhalation; injection into a muscle; by injection under the skin; intravenously; injection into the space around the spinal cord; transdermal; or via rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered by mouth, while the duration of its effect is 3–7 hours. Long-acting formulations of morphine are available as MS-Contin, Kadian, and other brand names as well as generically.

Thebaine (paramorphine), also known as codeine methyl enol ether, is an opiate alkaloid, its name coming from the Greek Θῆβαι, Thēbai (Thebes), an ancient city in Upper Egypt. A minor constituent of opium, thebaine is chemically similar to both morphine and codeine, but has stimulatory rather than depressant effects. At high doses, it causes convulsions similar to strychnine poisoning. The synthetic enantiomer (+)-thebaine does show analgesic effects apparently mediated through opioid receptors, unlike the inactive natural enantiomer (−)-thebaine. While thebaine is not used therapeutically, it is the main alkaloid extracted from Papaver bracteatum and can be converted industrially into a variety of compounds, including hydrocodone, hydromorphone, oxycodone, oxymorphone, nalbuphine, naloxone, naltrexone, buprenorphine, butorphanol and etorphine.
Oxycodone/aspirin is a combination drug marketed by Endo Pharmaceuticals. It is a tablet containing a mixture of 325 mg of aspirin and 4.8355 mg of oxycodone HCl ; it is an opioid/non-opioid combination used to treat moderate to moderately severe pain. The safety of the combination during pregnancy has not been established, although aspirin is generally contraindicated during pregnancy, and the drug has been placed in pregnancy category D. Inactive ingredients include D&C Yellow 10, FD&C Yellow 6, microcrystalline cellulose, and corn starch. Percodan was first marketed by DuPont Pharmaceuticals and prescribed in the United States in 1950. Once a widely prescribed painkiller, it has largely been replaced by alternative oxycodone compounds containing paracetamol (acetaminophen) instead of aspirin, such as Percocet.

Papaver somniferum, commonly known as the opium poppy or breadseed poppy, is a species of flowering plant in the family Papaveraceae. It is the species of plant from which both opium and poppy seeds are derived and is also a valuable ornamental plant, grown in gardens. Its native range is probably the eastern Mediterranean, but is now obscured by ancient introductions and cultivation, being naturalized across much of Europe and Asia.

Etorphine (M99) is a semi-synthetic opioid possessing an analgesic potency approximately 1,000–3,000 times that of morphine. It was first prepared in 1960 from oripavine, which does not generally occur in opium poppy extract but rather the related plants Papaver orientale and Papaver bracteatum. It was later reproduced in 1963 by a research group at MacFarlan Smith in Gorgie, Edinburgh, led by Kenneth Bentley. It can also be produced from thebaine.

Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others.
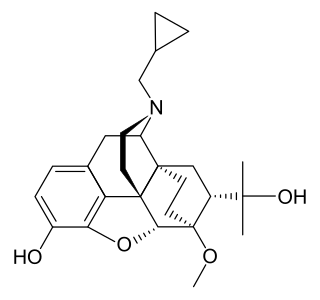
Diprenorphine, also known as diprenorfin, is a non-selective, high-affinity, weak partial agonist of the μ- (MOR), κ- (KOR), and δ-opioid receptor (DOR) which is used in veterinary medicine as an opioid antagonist. It is used to reverse the effects of super-potent opioid analgesics such as etorphine and carfentanil that are used for tranquilizing large animals. The drug is not approved for use in humans.

Dihydroetorphine was developed by K. W. Bentley at McFarlan-Smith in the 1960s and is a potent opioid analgesic used mainly in China. It is a derivative of the better-known opioid etorphine, a very potent veterinary painkiller and anesthetic medication used primarily for the sedation of large animals such as elephants, giraffes, and rhinos.

Oripavine is an opioid and the major metabolite of thebaine. It is the parent compound from which a series of semi-synthetic opioids are derived, which includes the compounds etorphine and buprenorphine. Although its analgesic potency is comparable to morphine, it is not used clinically due to its severe toxicity and low therapeutic index. Due to its use in manufacture of strong opioids, oripavine is a controlled substance in some jurisdictions.
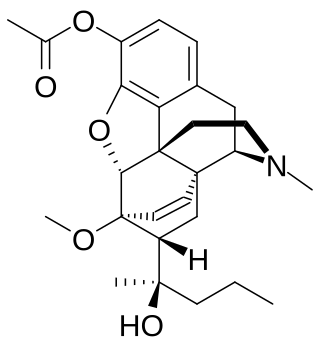
Acetorphine is a potent opioid analgesic, up to 8700 times stronger than morphine by weight. It is a derivative of the more well-known opioid etorphine, which is used as a very potent veterinary painkiller and anesthetic medication, primarily for the sedation of large animals such as elephants, giraffes and rhinos.
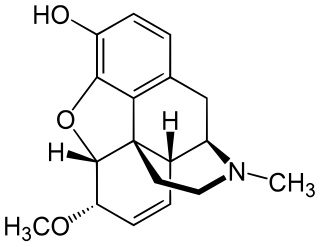
Heterocodeine (6-methoxymorphine) is an opiate derivative, the 6-methyl ether of morphine, and a structural isomer of codeine; it is called "hetero-" because it is the reverse isomer of codeine. Heterocodeine was first synthesised in 1932 and first patented in 1935. It can be made from morphine by selective methylation. Codeine is the natural mono-methyl ether, but must be metabolized for activity. In contrast the semi-synthetic mono-methyl ether, heterocodeine is a direct agonist. The 6,7,8,14 tetradehydro 3,6 methyl di-ether of morphine is thebaine.

7-PET is an opioid analgesic drug that has 300 times the potency of morphine by weight. It was discovered by K.W. Bentley and is related to the more well known oripavine derivative etorphine, which is used as a veterinary painkiller and anesthetic medication for the sedation of large animals such as elephants, giraffes, and rhinos. 7-PET itself has a 3-O-methyl ether which reduces potency, but the 3-OH derivative is around 2200 times more potent than morphine, almost the same potency as etorphine as a μ agonist, and unexpectedly the 3-hydrogen compound is also around the same potency of 2000 times morphine.

Papaver bracteatum, also known as the Iranian poppy or Persian poppy and the great scarlet poppy is a sturdy hardy perennial poppy with large deep red flowers up to 8 inches (20 cm) in diameter on stiff stalks up to 4 feet high with a prominent black spot near the base of the petals. It is closely related to the commonly cultivated oriental poppy, Papaver orientale and is sometimes recorded as the varietal form Papaver orientale var. bracteatum.

Poppy straw is derived from opium poppies that are harvested when fully mature and dried by mechanical means, minus the ripe poppy seeds. Opium poppy straw today can be one of several different things. It is what remains after the poppy seed harvest, that is, the dried stalks, stem and leaves of poppies grown for their seeds. The dried leaves and stalks are harvested after the seed pods have been used for traditional opium extraction. The field dried leaves, stalk and seed pod are used in commercial manufacture of morphine or other poppy alkaloid derived drugs, by first processing the material to make poppy straw separating the seeds then making concentrate of poppy straw, where no extraction using traditional methods of latex extraction has been made. The straw was originally considered an agricultural by-product of the mechanised poppy seed harvest, which was primarily grown for its edible and oil-producing seed. This changed in 1927 when János Kabay developed a chemical process to extract morphine from the crushed capsule. Concentrated poppy straw consisting mainly of the crushed capsule without the seeds soon became a valuable source of morphine. Today, concentrate of poppy straw is a major source of many opiates and other alkaloids. It is the source of 90% of the world supply of legal morphine and in some countries it also is a source of illegal morphine, which could be processed into illegal heroin.

Reticuline is a chemical compound found in a variety of plants including Lindera aggregata, Annona squamosa, and Ocotea fasciculata. It is based on the benzylisoquinoline structure.

The Bentley compounds are a class of semi-synthetic opioids that were first synthesized by K. W. Bentley by Diels-Alder reaction of thebaine with various dienophiles. The compounds are also known as thevinols, orvinols, or bridged oripavine derivatives, due to the characteristic 6,14-endo-ethano- or etheno-bridge and substitution at the 7α position. Buprenorphine and etorphine are perhaps the best known of the family, which was the first series of extremely potent μ-opioid agonists, with some compounds in the series having over many thousands of times the analgesic potency of morphine.
Extractas Bioscience is the largest opium poppy processing company in the Australian state of Tasmania. Approximately forty percent of the world's legal opiate crop is grown in Tasmania. Tasmanian Alkaloids was a subsidiary of the United States pharmaceutical company Johnson & Johnson, but was formerly—as of 1980—a subsidiary of Abbott Laboratories.

Synthesis of morphine-like alkaloids in chemistry describes the total synthesis of the natural morphinan class of alkaloids that includes codeine, morphine, oripavine, and thebaine and the closely related semisynthetic analogs methorphan, buprenorphine, hydromorphone, hydrocodone, isocodeine, naltrexone, nalbuphine, oxymorphone, oxycodone, and naloxone.
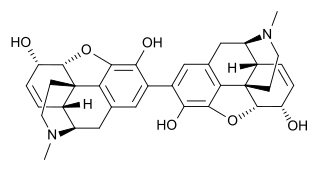
Pseudomorphine is an inactive, natural dimerisation product of the morphine molecule in tandem and thus a common impurity in morphine concentrations. It was first described by Pelletier in 1835.
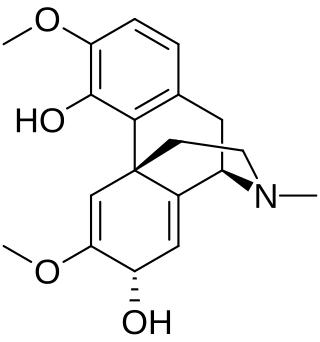
Salutaridinol is a modified benzyltetrahydroisoquinoline alkaloid with the formula C19H23NO4. It is produced in the secondary metabolism of the opium poppy Papaver somniferum (Papaveraceae) as an intermediate in the biosynthetic pathway that generates morphine. As an isoquinoline alkaloid, it is fundamentally derived from tyrosine as part of the shikimate pathway of secondary metabolism. Salutaridinol is a product of the enzyme salutaridine: NADPH 7-oxidoreductase and the substrate for the enzyme salutaridinol 7-O-acetyltransferase, which are two of the four enzymes in the morphine biosynthesis pathway that generates morphine from (R)-reticuline. Salutaridinol's unique position adjacent to two of the four enzymes in the morphine biosynthesis pathway gives it an important role in enzymatic, genetic, and synthetic biology studies of morphine biosynthesis. Salutaridinol levels are indicative of the flux through the morphine biosynthesis pathway and the efficacy of both salutaridine: NADPH 7-oxidoreductase and salutaridinol 7-O-acetyltransferase.

















