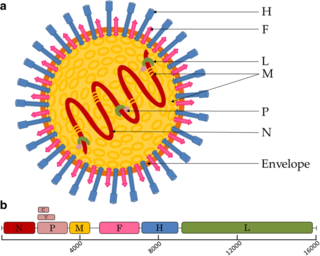
Paramyxoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates serve as natural hosts. Diseases associated with this family include measles, mumps, and respiratory tract infections. The family has four subfamilies, 17 genera, and 78 species, three genera of which are unassigned to a subfamily.

Filoviridae is a family of single-stranded negative-sense RNA viruses in the order Mononegavirales. Two members of the family that are commonly known are Ebola virus and Marburg virus. Both viruses, and some of their lesser known relatives, cause severe disease in humans and nonhuman primates in the form of viral hemorrhagic fevers.

Henipavirus is a genus of negative-strand RNA viruses in the family Paramyxoviridae, order Mononegavirales containing six established species, and numerous others still under study. Henipaviruses are naturally harboured by several species of small mammals, notably pteropid fruit bats, microbats of several species, and shrews. Henipaviruses are characterised by long genomes and a wide host range. Their recent emergence as zoonotic pathogens capable of causing illness and death in domestic animals and humans is a cause of concern.

Morbillivirus is a genus of viruses in the order Mononegavirales, in the family Paramyxoviridae. Humans, dogs, cats, cattle, seals, and cetaceans serve as natural hosts. This genus includes seven species. Diseases in humans associated with viruses classified in this genus include measles; in animals, they include acute febrile respiratory tract infection.

Rhabdoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates, invertebrates, plants, fungi and protozoans serve as natural hosts. Diseases associated with member viruses include rabies encephalitis caused by the rabies virus, and flu-like symptoms in humans caused by vesiculoviruses. The name is derived from Ancient Greek rhabdos, meaning rod, referring to the shape of the viral particles. The family has 40 genera, most assigned to three subfamilies.
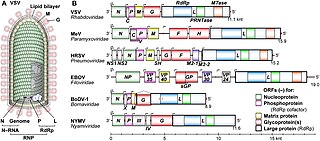
Mononegavirales is an order of negative-strand RNA viruses which have nonsegmented genomes. Some common members of the order are Ebola virus, human respiratory syncytial virus, measles virus, mumps virus, Nipah virus, and rabies virus. All of these viruses cause significant disease in humans. Many other important pathogens of nonhuman animals and plants are also in the group. The order includes eleven virus families: Artoviridae, Bornaviridae, Filoviridae, Lispiviridae, Mymonaviridae, Nyamiviridae, Paramyxoviridae, Pneumoviridae, Rhabdoviridae, Sunviridae, and Xinmoviridae.
Borna disease, also known as sad horse disease, is an infectious neurological syndrome of warm-blooded animals, caused by Borna disease viruses 1 and 2 (BoDV-1/2). BoDV-1/2 are neurotropic viruses of the species Mammalian 1 orthobornavirus, and members of the Bornaviridae family within the Mononegavirales order.
Bornaviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Horses, sheep, cattle, rodents, birds, reptiles, and humans serve as natural hosts. Diseases associated with bornaviruses include Borna disease, a fatal neurologic disease of mammals restricted to central Europe; and proventricular dilatation disease (PDD) in birds. Bornaviruses may cause encephalitis in mammals like horses or sheep. The family includes 11 species assigned to three genera.
Polyomaviridae is a family of viruses whose natural hosts are primarily mammals and birds. As of 2020, there are six recognized genera and 117 species, five of which are unassigned to a genus. 14 species are known to infect humans, while others, such as Simian Virus 40, have been identified in humans to a lesser extent. Most of these viruses are very common and typically asymptomatic in most human populations studied. BK virus is associated with nephropathy in renal transplant and non-renal solid organ transplant patients, JC virus with progressive multifocal leukoencephalopathy, and Merkel cell virus with Merkel cell cancer.

Lassa mammarenavirus (LASV) is an arenavirus that causes Lassa hemorrhagic fever, a type of viral hemorrhagic fever (VHF), in humans and other primates. Lassa mammarenavirus is an emerging virus and a select agent, requiring Biosafety Level 4-equivalent containment. It is endemic in West African countries, especially Sierra Leone, the Republic of Guinea, Nigeria, and Liberia, where the annual incidence of infection is between 300,000 and 500,000 cases, resulting in 5,000 deaths per year.

Nucleoproteins are proteins conjugated with nucleic acids. Typical nucleoproteins include ribosomes, nucleosomes and viral nucleocapsid proteins.
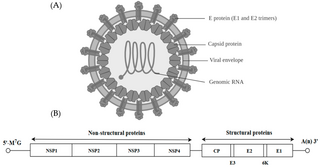
Alphavirus is a genus of RNA viruses, the sole genus in the Togaviridae family. Alphaviruses belong to group IV of the Baltimore classification of viruses, with a positive-sense, single-stranded RNA genome. There are 32 alphaviruses, which infect various vertebrates such as humans, rodents, fish, birds, and larger mammals such as horses, as well as invertebrates. Alphaviruses that could infect both vertebrates and arthropods are referred dual-host alphaviruses, while insect-specific alphaviruses such as Eilat virus and Yada yada virus are restricted to their competent arthropod vector. Transmission between species and individuals occurs mainly via mosquitoes, making the alphaviruses a member of the collection of arboviruses – or arthropod-borne viruses. Alphavirus particles are enveloped, have a 70 nm diameter, tend to be spherical, and have a 40 nm isometric nucleocapsid.

RNA-dependent RNA polymerase (RdRp) or RNA replicase is an enzyme that catalyzes the replication of RNA from an RNA template. Specifically, it catalyzes synthesis of the RNA strand complementary to a given RNA template. This is in contrast to typical DNA-dependent RNA polymerases, which all organisms use to catalyze the transcription of RNA from a DNA template.

A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea. Since Dmitri Ivanovsky's 1892 article describing a non-bacterial pathogen infecting tobacco plants and the discovery of the tobacco mosaic virus by Martinus Beijerinck in 1898, more than 9,000 virus species have been described in detail of the millions of types of viruses in the environment. Viruses are found in almost every ecosystem on Earth and are the most numerous type of biological entity. The study of viruses is known as virology, a subspeciality of microbiology.
In 2008, by pyrosequencing of cDNA from the brains of several parrots suffering from proventricular dilatation disease (PDD), Honkavuori et al. identified the presence of a novel bornavirus.
The species Lloviu cuevavirus is the taxonomic home of a virus that forms filamentous virion, Lloviu virus (LLOV). The species is included in the genus Cuevavirus. LLOV is a distant relative of the commonly known Ebola virus and Marburg virus.

Nyamiviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Ecdysozoa and birds serve as natural hosts. The name is a portmanteau of Nyamanini Pan and Midway Atoll and the suffix -viridae used to denote a virus family. There are seven genera in this family.
Perhabdovirus is a genus of viruses in the family Rhabdoviridae, order Mononegavirales. Fish serve as natural hosts. Diseases associated with viruses of this genus include: breathing and swimming problems.

Pneumoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Humans, cattle, and rodents serve as natural hosts. Respiratory tract infections are associated with member viruses such as human respiratory syncytial virus. There are five species in the family which are divided between the genera Metapneumovirus and Orthopneumovirus. The family used to be considered as a sub-family of Paramyxoviridae, but has been reclassified as of 2016.
Feline morbillivirus comes from the genus Morbillivirus, specifically influencing wild and domestic cats. The first report of a Feline morbillivirus outbreak occurred in Hong Kong in 2012. Approximately 10% of stray cats in Hong Kong and mainland China were reported to possess the virus at the time with additional infections found in Japan as well. 40% of cats tested in Japan were Fmo-PV positive and exhibited early symptoms of kidney failure. While the first cases of Feline morbillivirus were found in China, Hong Kong and Japan, the virus can also be found in Italy, Germany, and the United States. Feline morbillivirus exhibits a substantial amount of genetic diversity, yet cases in Japan and Hong Kong proved to have identical nucleotide sequences. It is also hypothesized that the morbillivirus has high adaptability due to its presence in multiple species. It is often found in dogs, cats, cattle, whales, dolphins, porpoises, and even humans. It likely originated from an ancestral version and underwent viral evolution to adapt to transmission in different species. Other common morbilliviruses include measles, rinderpest virus, canine distemper virus and peste des petits ruminants virus.