
Polyestradiol phosphate (PEP), sold under the brand name Estradurin, is an estrogen medication which is used primarily in the treatment of prostate cancer in men. It is also used in women to treat breast cancer, as a component of hormone therapy to treat low estrogen levels and menopausal symptoms, and as a component of feminizing hormone therapy for transgender women. It is given by injection into muscle once every four weeks.
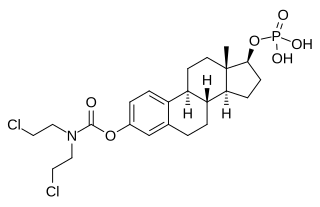
Estramustine phosphate (EMP), also known as estradiol normustine phosphate and sold under the brand names Emcyt and Estracyt, is a dual estrogen and chemotherapy medication which is used in the treatment of prostate cancer in men. It is taken multiple times a day by mouth or by injection into a vein.

Fosfestrol, sold under the brand name Honvan and also known as diethylstilbestrol diphosphate (DESDP), is an estrogen medication which is used in the treatment of prostate cancer in men. It is given by slow intravenous infusion once per day to once per week or by mouth once per day.
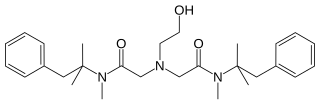
Oxetacaine is a potent local anesthetic. It is administered orally for the relief of pain associated with peptic ulcer disease or esophagitis. One example of such a product is Mucaine Gel, indicated for "rapid and effective relief in gastritis, esophagitis, hiatus hernia, heartburn of pregnancy and peptic ulcer". It is also used topically in the management of hemorrhoid pain. Oral oxetacaine preparations are available in several countries, including India, South Africa, Japan, Taiwan and Brazil, but not the United States. Unlike most local anesthetics, oxetacaine does not break down under strongly acidic conditions.
In enzymology, a dextrin dextranase is an enzyme that catalyzes the chemical reaction

Aquaporin-7 (AQP-7) is a protein that in humans is encoded by the AQP7 gene.

Estriol succinate, sold under the brand name Synapause among others, is an estrogen medication which is used in the treatment of menopausal symptoms. It is taken by mouth, in through the vagina, and by injection.
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol. Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability. In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat. Conversely, this is not the case with intravenous injection or oral administration. Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot. Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.
Phytoprogestogens, also known as phytoprogestins, are phytochemicals with progestogenic effects.

Diethylstilbestrol dipropionate (DESDP), or diethylstilbestrol dipropanoate, also known as stilboestrol dipropionate (BANM), is a synthetic nonsteroidal estrogen of the stilbestrol group that was formerly marketed widely throughout Europe. It is an ester of diethylstilbestrol with propionic acid, and is more slowly absorbed in the body than diethylstilbestrol. The medication has been said to be one of the most potent estrogens known.

Mytatrienediol, also known as 16α-methyl-16β-epiestriol 3-methyl ether or 16β-hydroxy-16α-methylestradiol 3-methyl ether, is a synthetic steroidal estrogen medication and an estrogen ether which was derived from estriol and was developed for clinical use in the late 1950s but was never marketed. It was investigated as a weak and mildly estrogenic medication for men to treat atherosclerosis, improve serum lipid profiles, and reduce the risk of myocardial infarction. However, while preclinical research supported the profile of mytatriendiol as a weak estrogen, the medication was found in clinical trials to produce estrogenic side effects including feminization, breast pain, and gynecomastia in men similarly and comparably to other estrogens such as ethinylestradiol and conjugated estrogens, and its side effects ultimately precluded its use. The medication was also studied to treat bone pain in patients with multiple myeloma, metastatic bone disease, and osteoporosis, with effectiveness seen.
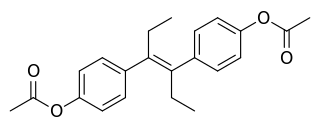
Diethylstilbestrol diacetate (DESDA) is a synthetic, nonsteroidal estrogen of the stilbestrol group and an ester of diethylstilbestrol (DES) that was introduced for clinical use in the 1940s and was formerly marketed but is now no longer available.

8,9-Dehydroestrone, or Δ8-estrone, also known as estra-1,3,5(10),8-tetraen-3-ol-17-one, is a naturally occurring estrogen found in horses which is closely related to equilin, equilenin, and estrone, and, as the 3-sulfate ester sodium salt, is a minor constituent (3.5%) of conjugated estrogens (Premarin). It produces 8,9-dehydro-17β-estradiol as an important active metabolite, analogously to conversion of estrone or estrone sulfate into estradiol. The compound was first described in 1997. In addition to 8,9-dehydroestrone and 8,9-dehydro-17β-estradiol, 8,9-dehydro-17α-estradiol is likely also to be present in conjugated estrogens, but has not been identified at this time.

MP-2001, also known as 2,3,4-trimethoxyestra-1,3,5(10)-trien-17β-ol or 2,4-dimethoxyestradiol 3-methyl ether, is a steroid and derivative of estradiol that was described in 1966 and is devoid of estrogenic activity but produces potent analgesic effects in animals. It was never marketed.
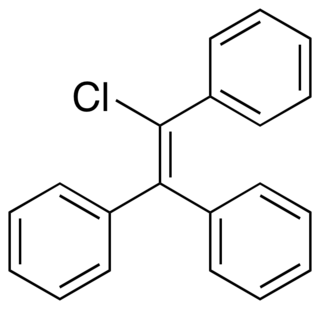
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.

17α-Ethynyl-3α-androstanediol, also known as 17α-ethynyl-5α-androstane-3α,17β-diol, is a synthetic androstane steroid and a 17α-substituted derivative of 3α-androstanediol which was never marketed. It was under development for the treatment of prostate cancer but was discontinued.

Polyestriol phosphate, sold under the brand names Gynäsan, Klimadurin, and Triodurin, is an estrogen medication which was previously used in menopausal hormone therapy and is no longer available.

17α-Ethynyl-3β-androstanediol is a synthetic estrogen and a 17α-substituted derivative of 3β-androstanediol which was never marketed.

Ethinylandrostenediol, also known as 17α-ethynyl-5-androstenediol, is a synthetic estrogen, progestogen, and androgen which was never marketed. It is the C17α ethynyl derivative of the androgen precursor and prohormone 5-androstenediol.
















