
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
Lactones are cyclic carboxylic esters are intramolecular esters derived from hydroxycarboxylic acids. They can be saturated or unsaturated. Some contain heteroatoms replacing one or more carbon atoms of the ring.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.
A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words lactone + amide.

Phendimetrazine is a stimulant drug of the morpholine chemical class used as an appetite suppressant.

The Martinet dioxindole synthesis was first reported in 1913 by J. Martinet. It is a chemical reaction in which a primary or secondary aniline or substituted aromatic amine is condensed with ethyl or methyl ester of mesoxalic acid to make a dioxindole in the absence of oxygen.
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal. It was first reported by Louis Bouveault and Gustave Louis Blanc in 1903. Bouveault and Blanc demonstrated the reduction of ethyl oleate and n-butyl oleate to oleyl alcohol. Modified versions of which were subsequently refined and published in Organic Syntheses.

The Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of taxol. Taxol is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the pacific yew.

The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996 two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis.
Azetidine is a saturated heterocyclic organic compound containing three carbon atoms and one nitrogen atom. It is a liquid at room temperature with a strong odor of ammonia and is strongly basic compared to most secondary amines.

Aluminium hydride is an inorganic compound with the formula AlH3. Alane and its derivatives are part of a family of common reducing reagents in organic synthesis based around group 13 hydrides. In solution—typically in ethereal solvents such tetrahydrofuran or diethyl ether—aluminium hydride forms complexes with Lewis bases, and reacts selectively with particular organic functional groups, and although it is not a reagent of choice, it can react with carbon-carbon multiple bonds. Given its density, and with hydrogen content on the order of 10% by weight, some forms of alane are, as of 2016, active candidates for storing hydrogen and so for power generation in fuel cell applications, including electric vehicles. As of 2006 it was noted that further research was required to identify an efficient, economical way to reverse the process, regenerating alane from spent aluminium product.

Lithium borohydride (LiBH4) is a borohydride and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages, being a stronger reducing agent and highly soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride.
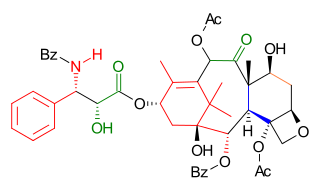
Wender Taxol total synthesis in organic chemistry describes a Taxol total synthesis by the group of Paul Wender at Stanford University published in 1997. This synthesis has much in common with the Holton Taxol total synthesis in that it is a linear synthesis starting from a naturally occurring compound with ring construction in the order A,B,C,D. The Wender effort is shorter by approximately 10 steps.
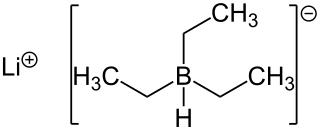
Lithium triethylborohydride is the organoboron compound with the formula LiEt3BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid but is typically marketed and used as a THF solution. The related reducing agent sodium triethylborohydride is commercially available as toluene solutions.

The Kuwajima Taxol total synthesis by the group of Isao Kuwajima of the Tokyo Institute of Technology is one of several efforts in taxol total synthesis published in the 1990s. The total synthesis of Taxol is considered a landmark in organic synthesis.

Reclazepam is a drug which is a benzodiazepine derivative. It has sedative and anxiolytic effects similar to those produced by other benzodiazepine derivatives, and has a short duration of action.

J-113,397 is an opioid drug which was the first compound found to be a highly selective antagonist for the nociceptin receptor, also known as the ORL-1 receptor. It is several hundred times selective for the ORL-1 receptor over other opioid receptors, and its effects in animals include preventing the development of tolerance to morphine, the prevention of hyperalgesia induced by intracerebroventricular administration of nociceptin, as well as the stimulation of dopamine release in the striatum, which increases the rewarding effects of cocaine, but may have clinical application in the treatment of Parkinson's disease.
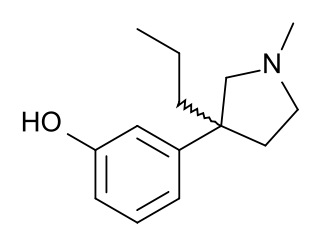
Profadol (CI-572) is an opioid analgesic which was developed in the 1960s by Parke-Davis. It acts as a mixed agonist-antagonist of the μ-opioid receptor. The analgetic potency is about the same as of pethidine (meperidine), the antagonistic effect is 1/50 of nalorphine.
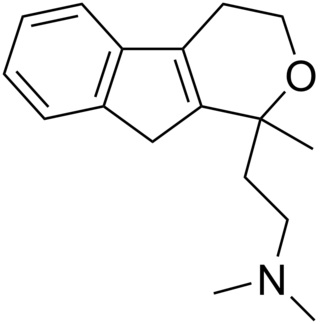
Pirandamine (AY-23,713) is a tricyclic derivative which acts as a selective serotonin reuptake inhibitor (SSRI). It was investigated in the 1970s as a potential antidepressant but clinical development was not commenced and it was never marketed. Pirandamine is structurally related to tandamine, which, in contrast, is a selective norepinephrine reuptake inhibitor.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.

















