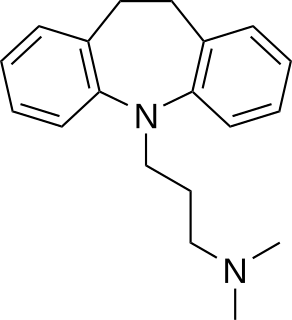
Tricyclic antidepressants (TCAs) are a class of medications that are used primarily as antidepressants, which is important for the management of depression. They are second-line drugs next to SSRIs. TCAs were discovered in the early 1950s and were marketed later in the decade. They are named after their chemical structure, which contains three rings of atoms. Tetracyclic antidepressants (TeCAs), which contain four rings of atoms, are a closely related group of antidepressant compounds.

Reuptake is the reabsorption of a neurotransmitter by a neurotransmitter transporter located along the plasma membrane of an axon terminal or glial cell after it has performed its function of transmitting a neural impulse.

Monoamine transporters (MATs) are protein structures that function as integral plasma-membrane transporters to regulate concentrations of extracellular monoamine neurotransmitters. Three major classes of MATs are responsible for the reuptake of their associated amine neurotransmitters. MATs are located just outside the synaptic cleft (peri-synaptically), transporting monoamine transmitter overflow from the synaptic cleft back to the cytoplasm of the pre-synaptic neuron. MAT regulation generally occurs through protein phosphorylation and posttranslational modification. Due to their significance in neuronal signaling, MATs are commonly associated with drugs used to treat mental disorders as well as recreational drugs. Compounds targeting MATs range from medications such as the wide variety of tricyclic antidepressants, selective serotonin reuptake inhibitors such as fluoxetine (Prozac) to stimulant medications such as methylphenidate (Ritalin) and amphetamine in its many forms and derivatives methamphetamine (Desoxyn) and lisdexamfetamine (Vyvanse). Furthermore, drugs such as MDMA and natural alkaloids such as cocaine exert their effects in part by their interaction with MATs, by blocking the transporters from mopping up dopamine, serotonin, and other neurotransmitters from the synapse.

Serotonin–noradrenaline reuptake inhibitors (SNRIs) are a class of antidepressant drugs that treat major depressive disorder (MDD), anxiety disorders, obsessive–compulsive disorder (OCD), social phobia, attention-deficit hyperactivity disorder (ADHD), chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and noradrenaline. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the more widely used selective serotonin reuptake inhibitors (SSRIs), which act upon serotonin only.
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.
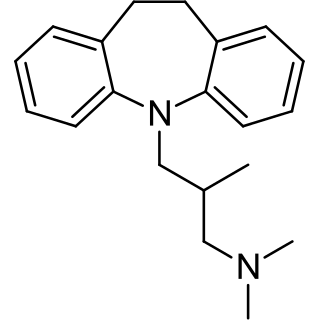
Trimipramine, sold under the brand name Surmontil among others, is a tricyclic antidepressant (TCA) which is used to treat depression. It has also been used for its sedative, anxiolytic, and weak antipsychotic effects in the treatment of insomnia, anxiety disorders, and psychosis, respectively. The drug is described as an atypical or "second-generation" TCA because, unlike other TCAs, it seems to be a fairly weak monoamine reuptake inhibitor. Similarly to other TCAs however, trimipramine does have antihistamine, antiserotonergic, antiadrenergic, antidopaminergic, and anticholinergic activities.

The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.
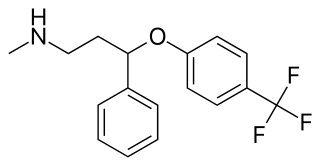
Fluoxetine, sold under the brand names Prozac and Sarafem among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used for the treatment of major depressive disorder, obsessive–compulsive disorder (OCD), bulimia nervosa, panic disorder, and premenstrual dysphoric disorder. It is also approved for treatment of major depressive disorder in adolescents and children 8 years of age and over. It has also been used to treat premature ejaculation. Fluoxetine is taken by mouth.

Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. Although RTI holds a strong position in this field, they are not the only researchers that have prepared these analogues. This research has spanned beyond the last couple decades, and has picked up its pace in recent times, creating numerous phenyltropanes as research into cocaine analogues garners interest to treat addiction.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission.

A reuptake inhibitor (RI) is a type of drug known as a reuptake modulator that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

Oxaprotiline, also known as hydroxymaprotiline, is a norepinephrine reuptake inhibitor of the tetracyclic antidepressant (TeCA) family that is related to maprotiline. Though investigated as an antidepressant, it was never marketed.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds.
A dopamine releasing agent (DRA) is a type of drug which induces the release of dopamine in the body and/or brain. No selective DRAs are currently known. Many releasing agents of both dopamine and norepinephrine and of serotonin, norepinephrine, and dopamine are known, however. Serotonin–dopamine releasing agents are much rarer and are not selective for monoamine release. Examples of NDRAs include amphetamine and methamphetamine, and an example of an SNDRA is MDMA. The most selective dopamine releaser is 4-methylaminorex, but it also has considerable activity as a norepinephrine releaser. These drugs are frequently used for recreational purposes and encountered as drugs of abuse.

JNJ-7925476 is a triple reuptake inhibitor antidepressant discovered by Johnson & Johnson, but never marketed.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

Decynium-22 is a cationic derivative of quinoline, and a potent inhibitor of the plasma membrane monoamine transporter (PMAT), as well as all members of the organic cation transporter (OCT) family in both human and rat cells. However, it has little effect on high affinity monoamine transporters such as the dopamine transporter and norepinephrine transporter.
Selective serotonin reuptake inhibitors, or serotonin-specific re-uptake inhibitor (SSRIs), are a class of chemical compounds that have contributed to the major advances as antidepressants where they have revolutionised the treatment of depression and other psychiatric disorders. The SSRIs are therapeutically useful in the treatment of panic disorder (PD), posttraumatic stress disorder (PTSD), social anxiety disorder, obsessive-compulsive disorder (OCD), premenstrual dysphoric disorder (PMDD) and anorexia. There is also clinical evidence of SSRIs efficiency in the treatment of the negative symptoms of schizophrenia and their ability to prevent cardiovascular diseases.
Selective norepinephrine reuptake inhibitors (sNRIs) are a class of drugs that have been marketed as antidepressants and are used for various mental disorders, mainly depression and attention-deficit hyperactivity disorder (ADHD). The norepinephrine transporter (NET) serves as the fundamental mechanism for the inactivation of noradrenergic signaling because of the NET termination in the reuptake of norepinephrine (NE). The selectivity and mechanism of action for the NRI drugs remain mostly unresolved and, to date, only a limited number of NRI-selective inhibitors are available. The first commercially available selective NRI was the drug reboxetine (Edronax), developed as a first-line therapy for major depressive disorder. Atomoxetine (Strattera) is another potent and selective NRI which is also effective and well tolerated for the treatment of ADHD in adults; it may also be a new treatment option for adults with ADHD, particularly for those patients at risk of substance abuse.














