Tricyclic antidepressants (TCAs) are a class of medications that are used primarily as antidepressants. TCAs were discovered in the early 1950s and were marketed later in the decade. They are named after their chemical structure, which contains three rings of atoms. Tetracyclic antidepressants (TeCAs), which contain four rings of atoms, are a closely related group of antidepressant compounds.

Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterised by executive dysfunction occasioning symptoms of inattention, hyperactivity, impulsivity and emotional dysregulation that are excessive and pervasive, impairing in multiple contexts, and otherwise age-inappropriate.

Methylphenidate, sold under the brand names Ritalin and Concerta among others, is a central nervous system (CNS) stimulant used medically to treat attention deficit hyperactivity disorder (ADHD) and, to a lesser extent, narcolepsy. It is a primary medication for ADHD ; it may be taken by mouth or applied to the skin, and different formulations have varying durations of effect, commonly ranging from 2–4 hours.

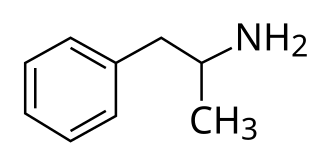
Dextroamphetamine is a potent central nervous system (CNS) stimulant and enantiomer of amphetamine that is prescribed for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. It is also used as an athletic performance and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant.

Clonidine, sold under the brand name Catapres among others, is an α2-adrenergic agonist medication used to treat high blood pressure, ADHD, drug withdrawal, menopausal flushing, diarrhea, spasticity, and certain pain conditions. It is used orally, by injection, or as a transdermal skin patch. Onset of action is typically within an hour with the effects on blood pressure lasting for up to eight hours.
Stimulant psychosis is a mental disorder characterized by psychotic symptoms. It involves and typically occurs following an overdose or several day 'binge' on psychostimulants; however, one study reported occurrences at regularly prescribed doses in approximately 0.1% of individuals within the first several weeks after starting amphetamine or methylphenidate therapy. Methamphetamine psychosis, or long-term effects of stimulant use in the brain, depend upon genetics and may persist for some time.
A paradoxical reaction is an effect of a chemical substance, such as a medical drug, that is opposite to what would usually be expected. An example of a paradoxical reaction is pain caused by a pain relief medication.

Atomoxetine, sold under the brand name Strattera, is a medication used to treat attention deficit hyperactivity disorder (ADHD) and, to a lesser extent, cognitive disengagement syndrome. It may be used alone or along with psychostimulants. It is also used as a cognitive and executive functioning enhancer to improve self-motivation, persistence, attention, inhibition, and working memory. Use of atomoxetine is only recommended for those who are at least six years old. It is taken orally. Atomoxetine is a selective norepinephrine reuptake inhibitor and is believed to work by increasing norepinephrine and dopamine levels in the brain. The effectiveness of atomoxetine is comparable to the commonly prescribed stimulant medication methylphenidate.
Adderall and Mydayis are trade names for a combination drug called mixed amphetamine salts containing four salts of amphetamine. The mixture is composed of equal parts racemic amphetamine and dextroamphetamine, which produces a (3:1) ratio between dextroamphetamine and levoamphetamine, the two enantiomers of amphetamine. Both enantiomers are stimulants, but differ enough to give Adderall an effects profile distinct from those of racemic amphetamine or dextroamphetamine, which are marketed as Evekeo and Dexedrine/Zenzedi, respectively. Adderall is used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. It is also used illicitly as an athletic performance enhancer, cognitive enhancer, appetite suppressant, and recreationally as a euphoriant. It is a central nervous system (CNS) stimulant of the phenethylamine class.
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.
Adult Attention Deficit Hyperactivity Disorder is the persistence of attention deficit hyperactivity disorder (ADHD) in adults. It is a neurodevelopmental disorder, meaning symptoms must have been present in childhood except for when ADHD occurs after traumatic brain injury. Specifically, for ADHD, multiple symptoms must have been present before the age of 12, according to DSM-5 diagnostic criteria. This cutoff age of 12 is a change from the previous requirement of symptom onset before the age of 7 in the DSM-IV to add flexibility in the diagnosis of adults. ADHD was previously thought to be a childhood disorder that improved with age, but recent research has disproved this. Approximately two-thirds of childhood cases of ADHD continue into adulthood, with varying degrees of symptom severity that change over time, and continue to significantly affect individuals' daily functioning in multiple domains.

Pemoline, sold under the brand name Cylert among others, is a stimulant medication which has been used in the treatment of attention-deficit hyperactivity disorder (ADHD) and narcolepsy. It has been discontinued in most countries due to rare but serious problems with liver toxicity. The medication was taken by mouth.

Viloxazine, sold under the brand name Qelbree and formerly as Vivalan among others, is a selective norepinephrine reuptake inhibitor medication which is used in the treatment of attention deficit hyperactivity disorder (ADHD) in children and adults. It was marketed for almost 30 years as an antidepressant for the treatment of depression before being discontinued and subsequently repurposed as a treatment for ADHD. Viloxazine is taken orally. It was used as an antidepressant in an immediate-release form and is used in ADHD in an extended-release form.
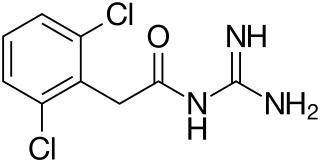
Guanfacine, sold under the brand name Tenex (immediate-release) and Intuniv (extended-release) among others, is an oral alpha-2a agonist medication used to treat attention deficit hyperactivity disorder (ADHD) and high blood pressure. Guanfacine is FDA-approved for monotherapy treatment of ADHD, as well as being used for augmentation of other treatments, such as stimulants. Guanfacine is also used off-label to treat tic disorders, anxiety disorders and PTSD.
Attention deficit hyperactivity disorder predominantly inattentive, is one of the three presentations of attention deficit hyperactivity disorder (ADHD). In 1987–1994, there were no subtypes and thus it was not distinguished from hyperactive ADHD in the Diagnostic and Statistical Manual (DSM-III-R).

Despite the scientifically well-established nature of attention deficit hyperactivity disorder (ADHD), its diagnosis, and its treatment, each of these has been controversial since the 1970s. The controversies involve clinicians, teachers, policymakers, parents, and the media. Positions range from the view that ADHD is within the normal range of behavior to the hypothesis that ADHD is a genetic condition. Other areas of controversy include the use of stimulant medications in children, the method of diagnosis, and the possibility of overdiagnosis. In 2009, the National Institute for Health and Care Excellence, while acknowledging the controversy, stated that the current treatments and methods of diagnosis are based on the dominant view of the academic literature.

Lisdexamfetamine, most commonly sold under the brand name Vyvanse and Elvanse among others, is a stimulant medication that is used to treat attention deficit hyperactivity disorder (ADHD) in children and adults, and for moderate-to-severe binge eating disorder in adults. Lisdexamfetamine is taken by mouth. Its effects generally begin within two hours and last for up to 14 hours. In the United Kingdom, it is usually less preferred to methylphenidate for the treatment of children.
Attention deficit hyperactivity disorder management options are evidence-based practices with established treatment efficacy for ADHD.
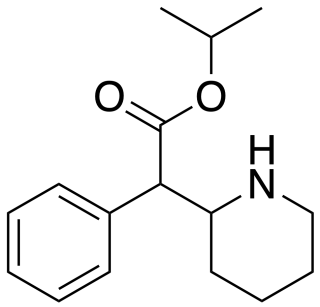
Isopropylphenidate is a piperidine based stimulant drug, closely related to methylphenidate, but with the methyl ester replaced by an isopropyl ester. It has similar effects to methylphenidate but with a longer duration of action, and was banned in the UK as a Temporary Class Drug from April 2015 following its unapproved sale as a designer drug.

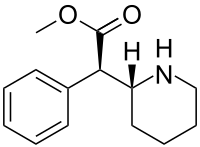
Serdexmethylphenidate/dexmethylphenidate, sold under the brand name Azstarys, is a fixed-dose combination medication containing serdexmethylphenidate, a prodrug of dexmethylphenidate, and dexmethylphenidate, a d-threo enantiomer of racemic methylphenidate, which is used to treat attention deficit hyperactivity disorder (ADHD) in people aged six years and older.