Acetanilide is an odourless solid chemical of leaf or flake-like appearance. It is also known as N-phenylacetamide, acetanil, or acetanilid, and was formerly known by the trade name Antifebrin.

Armodafinil (trade name Nuvigil) is the enantiopure compound of the eugeroic modafinil (Provigil). It consists of only the (R)-(−)-enantiomer of the racemic modafinil. Armodafinil is produced by the pharmaceutical company Cephalon Inc. and was approved by the U.S. Food and Drug Administration (FDA) in June 2007. In 2016, the FDA granted Mylan rights for the first generic version of Cephalon's Nuvigil to be marketed in the U.S.
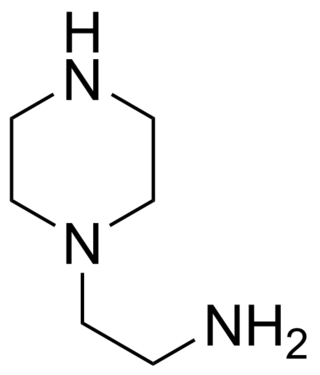
Aminoethylpiperazine (AEP) is a derivative of piperazine. This ethyleneamine contains three nitrogen atoms; one primary, one secondary and one tertiary. It is a corrosive organic liquid and can cause second or third degree burns. Aminoethylpiperazine can also cause pulmonary edema as a result of inhalation. It is REACH and TSCA registered.

Nitenpyram is a chemical frequently used as an insecticide in agriculture and veterinary medicine. The compound is an insect neurotoxin belonging to the class of neonicotinoids which works by blocking neural signaling of the central nervous system. It does so by binding irreversibly to the nicotinic acetylcholine receptor (nACHr) causing a stop of the flow of ions in the postsynaptic membrane of neurons leading to paralysis and death. Nitenpyram is highly selective towards the variation of the nACHr which insects possess, and has seen extensive use in targeted, insecticide applications.
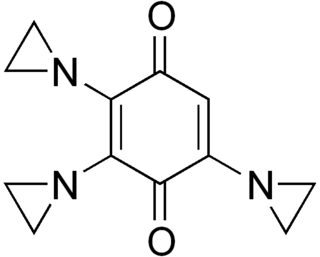
Triaziquone is a drug used in chemotherapy.
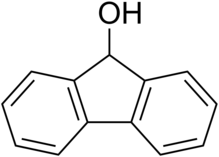
1-Naphthol, or α-naphthol, is a organic compound with the formula C10H7OH. It is a fluorescent white solid. 1-Naphthol differs from its isomer 2-naphthol by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol. Both isomers are soluble in simple organic solvents. They are precursors to a variety of useful compounds.

Eugeroics, also known as wakefulness-promoting agents and wakefulness-promoting drugs, are a class of drugs that promote wakefulness and alertness. They are medically indicated for the treatment of certain sleep disorders including excessive daytime sleepiness (EDS) in narcolepsy or obstructive sleep apnea (OSA). Eugeroics are also often prescribed off-label for the treatment of EDS in idiopathic hypersomnia. In contrast to classical psychostimulants, such as methylphenidate and amphetamine, which are also used in the treatment of these disorders, eugeroics typically do not produce marked euphoria, and, consequently, have a lower addictive potential.

Demeton, sold as an amber oily liquid with a sulphur like odour under the name Systox, is an organophosphate derivative causing irritability and shortness of breath to individuals repeatedly exposed. It was used as a phosphorothioate insecticide and acaricide and has the chemical formula C8H19O3PS2. Although it was previously used as an insecticide, it is now largely obsolete due to its relatively high toxicity to humans. Demeton consists of two components, demeton-S and demeton-O in a ratio of approximately 2:1 respectively. The chemical structure of demeton is closely related to military nerve agents such as VX and a derivative with one of the ethoxy groups replaced by methyl was investigated by both the US and Soviet chemical-weapons programs under the names V-sub x and GD-7.

The benzodioxans are a group of isomeric chemical compounds with the molecular formula C8H8O2. There are three isomers of benzodioxan, as the second atom of oxygen of the dioxane can be in a second, third or fourth position: 1,2-dioxane, 1,3-dioxane and 1,4-dioxane, which respectively give 1,2-benzodioxan, 1,3-benzodioxan and 1,4-benzodioxan.
Neoendorphins are a group of endogenous opioid peptides derived from the proteolytic cleavage of prodynorphin. They include α-neoendorphin and β-neoendorphin. The α-neoendorphin is present in greater amounts in the brain than β-neoendorphin. Both are products of the dynorphin gene, which also expresses dynorphin A, dynorphin A-(1-8), and dynorphin B. These opioid neurotransmitters are especially active in Central Nervous System receptors, whose primary function is pain sensation. These peptides all have the consensus amino acid sequence of Try-Gly-Gly-Phe-Met (met-enkephalin) or Tyr-Gly-Gly-Phe-Leu ( leu-enkephalin). Binding of neoendorphins to opioid receptors (OPR), in the dorsal root ganglion (DRG) neurons results in the reduction of time of calcium-dependent action potential. The α-neoendorphins bind OPRD1(delta), OPRK1(kappa), and OPRM1 (mu) and β-neoendorphin bind OPRK1.
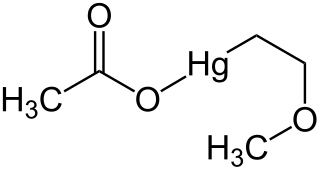
Methoxyethylmercuric acetate is a chemical compound formerly used as a pesticide for seeds of cotton and small grains. It is highly toxic, and can pose a threat to the brain and central nervous system.

Triazofos is a chemical compound used in acaricides, insecticides, and nematicides.

Dioscorine is an alkaloid toxin isolated from the tubers of tropical yam on several continents. It has been used as a monkey poison in some African countries, and as an arrow poison to aid in hunting in several parts of Asia. It was first isolated from Dioscorea hirsute by Boorsma in 1894 and obtained in a crystalline form by Schutte in 1897, and has since been found in other Dioscorea species. Dioscorine is a neurotoxin that acts by blocking the nicotinic acetylcholine receptor. Dioscorine is generally isolated in tandem with other alkaloids such as dioscin but is usually the most potent toxin in the mixture. It is a convulsant, producing symptoms similar to picrotoxin, with which it shares a similar mechanism of action.

Buprofezin is an insecticide used for control of insect pests such as mealybugs, leafhoppers and whitefly on vegetable crops. It is a growth regulator, acting as an inhibitor of chitin synthesis. It is banned in some countries due to its negative environmental impacts, being especially toxic to aquatic organisms as well as non-target insects, though is of low toxicity to humans and other mammals.

Pregnenolone acetate, also known as pregn-5-en-3β-ol-20-one 3β-acetate, is a synthetic pregnane steroid and an ester of pregnenolone which is described as a glucocorticoid and as a skin-conditioning and skin anti-aging agent. It has been reported to reduce wrinkles in elderly women when applied in the form of a 0.5% topical cream, effects which were suggested to be due to improved hydration of the skin. Pregnenolone acetate has been marketed in France in a topical cream containing 1% pregnenolone acetate and 10% "sex hormone" for the treatment of premature skin aging but was withdrawn from the market in 1992. Although the medication has been described by some sources as a glucocorticoid, other authors have stated that systemic pregnenolone acetate has no undesirable metabolic or toxic effects even at high doses.
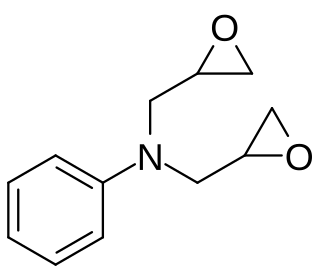
Diglycidyl aniline is an aromatic organic chemical in the glycidyl compound family. It is used to reduce the viscosity of epoxy resin systems. It has the empirical formula C12H15NO2 and the IUPAC name is N,N-bis(oxiran-2-ylmethyl)aniline. The CAS number is 2095-06-9. It is REACH registered in Europe with the EC number 218-259-5. A key use is in the viscosity reduction of epoxy resin systems functioning as a reactive diluent.
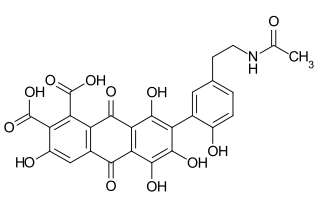
Laccaic acids or laccainic acids are a group of five anthraquinone derivatives, designated A through E, which are components of the red shellac obtained from the insect Kerria lacca, similar to carminic acid and kermesic acid. This article focuses primarily on laccaic acid A (LCA).

Coriamyrtin is a toxic γ-lactone naturally present in a multitude of plants.