Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. Endocytosis includes pinocytosis and phagocytosis. It is a form of active transport.

A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids and sex steroids. Within those two classes are five types according to the receptors to which they bind: glucocorticoids and mineralocorticoids and androgens, estrogens, and progestogens. Vitamin D derivatives are a sixth closely related hormone system with homologous receptors. They have some of the characteristics of true steroids as receptor ligands.
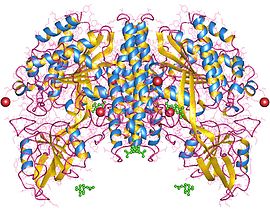
Transferrins are glycoproteins found in vertebrates which bind to and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded by the TF gene and produced as a 76 kDa glycoprotein.

Endosomes are a collection of intracellular sorting organelles in eukaryotic cells. They are parts of endocytic membrane transport pathway originating from the trans Golgi network. Molecules or ligands internalized from the plasma membrane can follow this pathway all the way to lysosomes for degradation or can be recycled back to the cell membrane in the endocytic cycle. Molecules are also transported to endosomes from the trans Golgi network and either continue to lysosomes or recycle back to the Golgi apparatus.
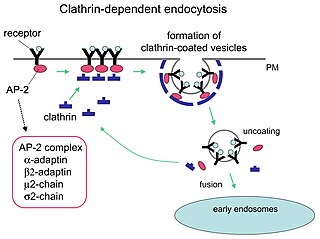
Receptor-mediated endocytosis (RME), also called clathrin-mediated endocytosis, is a process by which cells absorb metabolites, hormones, proteins – and in some cases viruses – by the inward budding of the plasma membrane (invagination). This process forms vesicles containing the absorbed substances and is strictly mediated by receptors on the surface of the cell. Only the receptor-specific substances can enter the cell through this process.

Omigapil is a drug that was developed by Novartis and tested in clinical trials for its ability to help treat Parkinson's disease (PD) and amyotrophic lateral sclerosis (ALS). The development for PD and ALS have been terminated due to lack of benefit, but Santhera Pharmaceuticals bought the compound for development for the treatment of congenital muscular dystrophy (CMD).

Human iron metabolism is the set of chemical reactions that maintain human homeostasis of iron at the systemic and cellular level. Iron is both necessary to the body and potentially toxic. Controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism, because iron is essential for red blood cells, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron-deficiency anemia.
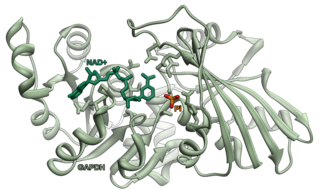
Glyceraldehyde 3-phosphate dehydrogenase is an enzyme of about 37kDa that catalyzes the sixth step of glycolysis and thus serves to break down glucose for energy and carbon molecules. In addition to this long established metabolic function, GAPDH has recently been implicated in several non-metabolic processes, including transcription activation, initiation of apoptosis, ER to Golgi vesicle shuttling, and fast axonal, or axoplasmic transport. In sperm, a testis-specific isoenzyme GAPDHS is expressed.
Transcytosis is a type of transcellular transport in which various macromolecules are transported across the interior of a cell. Macromolecules are captured in vesicles on one side of the cell, drawn across the cell, and ejected on the other side. Examples of macromolecules transported include IgA, transferrin, and insulin. While transcytosis is most commonly observed in epithelial cells, the process is also present elsewhere. Blood capillaries are a well-known site for transcytosis, though it occurs in other cells, including neurons, osteoclasts and M cells of the intestine.

Fructose-bisphosphate aldolase, often just aldolase, is an enzyme catalyzing a reversible reaction that splits the aldol, fructose 1,6-bisphosphate, into the triose phosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P). Aldolase can also produce DHAP from other (3S,4R)-ketose 1-phosphates such as fructose 1-phosphate and sedoheptulose 1,7-bisphosphate. Gluconeogenesis and the Calvin cycle, which are anabolic pathways, use the reverse reaction. Glycolysis, a catabolic pathway, uses the forward reaction. Aldolase is divided into two classes by mechanism.

The iron-responsive element-binding proteins, also known as IRE-BP, IRBP, IRP and IFR , bind to iron-responsive elements (IREs) in the regulation of human iron metabolism.

Iron is an important biological element. It is used in both the ubiquitous Iron-sulfur proteins and in Vertebrates it is used in Hemoglobin which is essential for Blood and oxygen transport.

Transferrin receptor 2 (TfR2) is a protein that in humans is encoded by the TFR2 gene. This protein is involved in the uptake of transferrin-bound iron into cells by endocytosis, although its role is minor compared to transferrin receptor 1.

Transferrin receptor protein 1 (TfR1), also known as Cluster of Differentiation 71 (CD71), is a protein that in humans is encoded by the TFRC gene. TfR1 is required for iron import from transferrin into cells by endocytosis.

Protein kinase C iota type is an enzyme that in humans is encoded by the PRKCI gene.

Glyceraldehyde-3-phosphate dehydrogenase, spermatogenic or glyceraldehyde-3-phosphate dehydrogenase, testis-specific is an enzyme that in humans is encoded by the GAPDHS gene.
Soluble transferrin receptor conventionally refers to the cleaved extracellular portion of transferrin receptor 1 that is released into serum. This receptor is a protein dimer of two identical subunits, linked together by two pairs of disulfide bonds. Its molecular mass 190,000 Dalton.

Protein moonlighting is a phenomenon by which a protein can perform more than one function. It is an excellent example of gene sharing.

Tyrosine phosphorylation is the addition of a phosphate (PO43−) group to the amino acid tyrosine on a protein. It is one of the main types of protein phosphorylation. This transfer is made possible through enzymes called tyrosine kinases. Tyrosine phosphorylation is a key step in signal transduction and the regulation of enzymatic activity.

Aconitase 1, soluble is a protein that in humans is encoded by the ACO1 gene.