
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information.
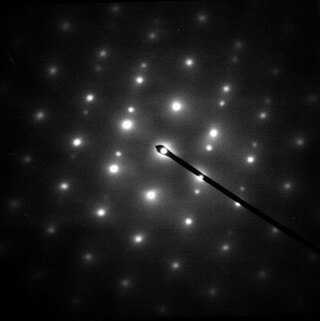
Electron diffraction refers to changes in the direction of electron beams due to interactions with atoms. Close to the atoms the changes are described as Fresnel diffraction; far away they are called Fraunhofer diffraction. The resulting map of the directions of the electrons far from the sample is called a diffraction pattern, see for instance Figure 1. These patterns are similar to x-ray and neutron diffraction patterns, and are used to study the atomic structure of gases, liquids, surfaces and bulk solids. Electron diffraction also plays a major role in the contrast of images in electron microscopes.
In physics and chemistry, Bragg's law, Wulff–Bragg's condition or Laue–Bragg interference, a special case of Laue diffraction, gives the angles for coherent scattering of waves from a large crystal lattice. It encompasses the superposition of wave fronts scattered by lattice planes, leading to a strict relation between wavelength and scattering angle, or else to the wavevector transfer with respect to the crystal lattice. Such law had initially been formulated for X-rays upon crystals. However, it applies to all sorts of quantum beams, including neutron and electron waves at atomic distances if there are a large number of atoms, as well as visible light with artificial periodic microscale lattices.
Reflection high-energy electron diffraction (RHEED) is a technique used to characterize the surface of crystalline materials. RHEED systems gather information only from the surface layer of the sample, which distinguishes RHEED from other materials characterization methods that also rely on diffraction of high-energy electrons. Transmission electron microscopy, another common electron diffraction method samples mainly the bulk of the sample due to the geometry of the system, although in special cases it can provide surface information. Low-energy electron diffraction (LEED) is also surface sensitive, but LEED achieves surface sensitivity through the use of low energy electrons.

In physics, the reciprocal lattice represents the Fourier transform of another lattice. The direct lattice or real lattice is a periodic function in physical space, such as a crystal system. The reciprocal lattice exists in the mathematical space of spatial frequencies, known as reciprocal space or k space, where refers to the wavevector.
In solid-state physics, the electronic band structure of a solid describes the range of energy levels that electrons may have within it, as well as the ranges of energy that they may not have.

Miller indices form a notation system in crystallography for lattice planes in crystal (Bravais) lattices.
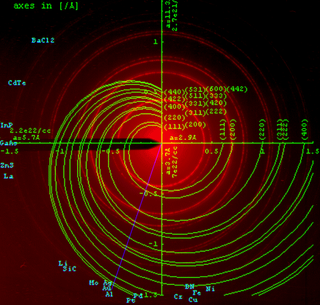
Powder diffraction is a scientific technique using X-ray, neutron, or electron diffraction on powder or microcrystalline samples for structural characterization of materials. An instrument dedicated to performing such powder measurements is called a powder diffractometer.
In particle physics, wave mechanics and optics, momentum transfer is the amount of momentum that one particle gives to another particle. It is also called the scattering vector as it describes the transfer of wavevector in wave mechanics.

In physics, a Bragg plane is a plane in reciprocal space which bisects a reciprocal lattice vector, , at right angles. The Bragg plane is defined as part of the Von Laue condition for diffraction peaks in x-ray diffraction crystallography.
The Debye–Waller factor (DWF), named after Peter Debye and Ivar Waller, is used in condensed matter physics to describe the attenuation of x-ray scattering or coherent neutron scattering caused by thermal motion. It is also called the B factor, atomic B factor, or temperature factor. Often, "Debye–Waller factor" is used as a generic term that comprises the Lamb–Mössbauer factor of incoherent neutron scattering and Mössbauer spectroscopy.
X-ray crystal truncation rod scattering is a powerful method in surface science, based on analysis of surface X-ray diffraction (SXRD) patterns from a crystalline surface.

Low-energy electron diffraction (LEED) is a technique for the determination of the surface structure of single-crystalline materials by bombardment with a collimated beam of low-energy electrons (30–200 eV) and observation of diffracted electrons as spots on a fluorescent screen.
In condensed matter physics and crystallography, the static structure factor is a mathematical description of how a material scatters incident radiation. The structure factor is a critical tool in the interpretation of scattering patterns obtained in X-ray, electron and neutron diffraction experiments.

In physics, the atomic form factor, or atomic scattering factor, is a measure of the scattering amplitude of a wave by an isolated atom. The atomic form factor depends on the type of scattering, which in turn depends on the nature of the incident radiation, typically X-ray, electron or neutron. The common feature of all form factors is that they involve a Fourier transform of a spatial density distribution of the scattering object from real space to momentum space. For an object with spatial density distribution, , the form factor, , is defined as
Helium atom scattering (HAS) is a surface analysis technique used in materials science. HAS provides information about the surface structure and lattice dynamics of a material by measuring the diffracted atoms from a monochromatic helium beam incident on the sample.
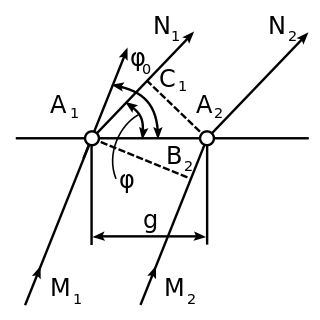
In crystallography and solid state physics, the Laue equations relate incoming waves to outgoing waves in the process of elastic scattering, where the photon energy or light temporal frequency does not change upon scattering by a crystal lattice. They are named after physicist Max von Laue (1879–1960).
The multislice algorithm is a method for the simulation of the elastic interaction of an electron beam with matter, including all multiple scattering effects. The method is reviewed in the book by Cowley, and also the work by Ishizuka. The algorithm is used in the simulation of high resolution transmission electron microscopy micrographs, and serves as a useful tool for analyzing experimental images. This article describes some relevant background information, the theoretical basis of the technique, approximations used, and several software packages that implement this technique. Some of the advantages and limitations of the technique and important considerations that need to be taken into account are described.
In crystallography, mosaicity is a measure of the spread of crystal plane orientations. A mosaic crystal is an idealized model of an imperfect crystal, imagined to consist of numerous small perfect crystals (crystallites) that are to some extent randomly misoriented. Empirically, mosaicities can be determined by measuring rocking curves. Diffraction by mosaics is described by the Darwin–Hamilton equations.
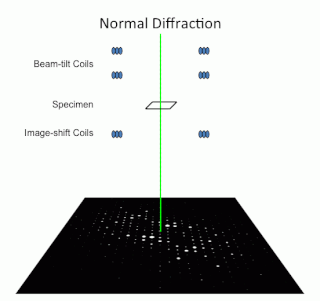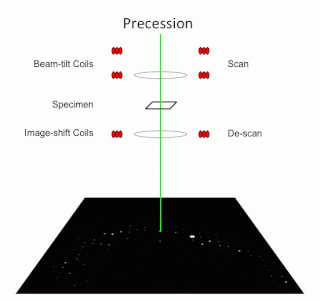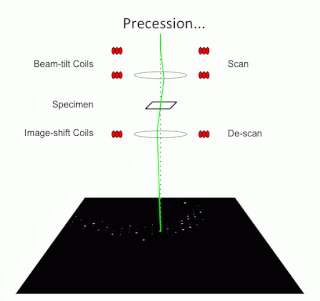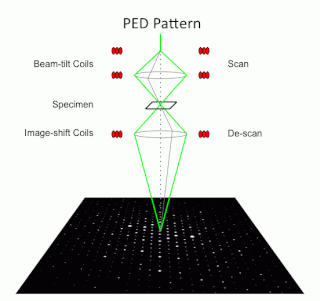
Precession electron diffraction (PED) is a specialized method to collect electron diffraction patterns in a transmission electron microscope (TEM). By rotating (precessing) a tilted incident electron beam around the central axis of the microscope, a PED pattern is formed by integration over a collection of diffraction conditions. This produces a quasi-kinematical diffraction pattern that is more suitable as input into direct methods algorithms to determine the crystal structure of the sample.



















