Related Research Articles

Malaria is a mosquito-borne infectious disease that affects humans and other vertebrates. Human malaria causes symptoms that typically include fever, fatigue, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. Symptoms usually begin 10 to 15 days after being bitten by an infected Anopheles mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria.
Antimalarial medications or simply antimalarials are a type of antiparasitic chemical agent, often naturally derived, that can be used to treat or to prevent malaria, in the latter case, most often aiming at two susceptible target groups, young children and pregnant women. As of 2018, modern treatments, including for severe malaria, continued to depend on therapies deriving historically from quinine and artesunate, both parenteral (injectable) drugs, expanding from there into the many classes of available modern drugs. Incidence and distribution of the disease is expected to remain high, globally, for many years to come; moreover, known antimalarial drugs have repeatedly been observed to elicit resistance in the malaria parasite—including for combination therapies featuring artemisinin, a drug of last resort, where resistance has now been observed in Southeast Asia. As such, the needs for new antimalarial agents and new strategies of treatment remain important priorities in tropical medicine. As well, despite very positive outcomes from many modern treatments, serious side effects can impact some individuals taking standard doses.

Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in malaria. During this infection, some parasites are picked up by a blood-feeding insect, continuing the life cycle.

Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases. P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer and is classified as a Group 2A (probable) carcinogen.

CD36, also known as platelet glycoprotein 4, fatty acid translocase (FAT), scavenger receptor class B member 3 (SCARB3), and glycoproteins 88 (GP88), IIIb (GPIIIB), or IV (GPIV) is a protein that in humans is encoded by the CD36 gene. The CD36 antigen is an integral membrane protein found on the surface of many cell types in vertebrate animals. It imports fatty acids inside cells and is a member of the class B scavenger receptor family of cell surface proteins. CD36 binds many ligands including collagen, thrombospondin, erythrocytes parasitized with Plasmodium falciparum, oxidized low density lipoprotein, native lipoproteins, oxidized phospholipids, and long-chain fatty acids.

Plasmodium malariae is a parasitic protozoan that causes malaria in humans. It is one of several species of Plasmodium parasites that infect other organisms as pathogens, also including Plasmodium falciparum and Plasmodium vivax, responsible for most malarial infection. Found worldwide, it causes a so-called "benign malaria", not nearly as dangerous as that produced by P. falciparum or P. vivax. The signs include fevers that recur at approximately three-day intervals – a quartan fever or quartan malaria – longer than the two-day (tertian) intervals of the other malarial parasite.

Merozoitesurface proteins are both integral and peripheral membrane proteins found on the surface of a merozoite, an early life cycle stage of a protozoan. Merozoite surface proteins, or MSPs, are important in understanding malaria, a disease caused by protozoans of the genus Plasmodium. During the asexual blood stage of its life cycle, the malaria parasite enters red blood cells to replicate itself, causing the classic symptoms of malaria. These surface protein complexes are involved in many interactions of the parasite with red blood cells and are therefore an important topic of study for scientists aiming to combat malaria.
Antigenic variation or antigenic alteration refers to the mechanism by which an infectious agent such as a protozoan, bacterium or virus alters the proteins or carbohydrates on its surface and thus avoids a host immune response, making it one of the mechanisms of antigenic escape. It is related to phase variation. Antigenic variation not only enables the pathogen to avoid the immune response in its current host, but also allows re-infection of previously infected hosts. Immunity to re-infection is based on recognition of the antigens carried by the pathogen, which are "remembered" by the acquired immune response. If the pathogen's dominant antigen can be altered, the pathogen can then evade the host's acquired immune system. Antigenic variation can occur by altering a variety of surface molecules including proteins and carbohydrates. Antigenic variation can result from gene conversion, site-specific DNA inversions, hypermutation, or recombination of sequence cassettes. The result is that even a clonal population of pathogens expresses a heterogeneous phenotype. Many of the proteins known to show antigenic or phase variation are related to virulence.

Malaria culture is a method for growing malaria parasites outside the body, i.e., in an ex vivo environment. Although attempts for propagation of the parasites outside of humans or animal models reach as far back as 1912, the success of the initial attempts was limited to one or just a few cycles. The first successful continuous culture was established in 1976. Initial hopes that the ex vivo culture would lead quickly to the discovery of a vaccine were premature. However, the development of new drugs was greatly facilitated.
Malaria prophylaxis is the preventive treatment of malaria. Several malaria vaccines are under development.
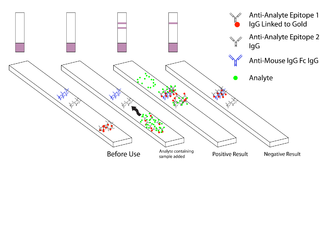
Malaria antigen detection tests are a group of commercially available rapid diagnostic tests of the rapid antigen test type that allow quick diagnosis of malaria by people who are not otherwise skilled in traditional laboratory techniques for diagnosing malaria or in situations where such equipment is not available. There are currently over 20 such tests commercially available. The first malaria antigen suitable as target for such a test was a soluble glycolytic enzyme Glutamate dehydrogenase. None of the rapid tests are currently as sensitive as a thick blood film, nor as cheap. A major drawback in the use of all current dipstick methods is that the result is essentially qualitative. In many endemic areas of tropical Africa, however, the quantitative assessment of parasitaemia is important, as a large percentage of the population will test positive in any qualitative assay.
Malaria vaccines are vaccines that prevent malaria, a mosquito-borne infectious disease which annually affects an estimated 247 million people worldwide and causes 619,000 deaths. The first approved vaccine for malaria is RTS,S, known by the brand name Mosquirix. As of April 2023, the vaccine has been given to 1.5 million children living in areas with moderate-to-high malaria transmission. It requires at least three doses in infants by age 2, and a fourth dose extends the protection for another 1–2 years. The vaccine reduces hospital admissions from severe malaria by around 30%.

Haemozoin is a disposal product formed from the digestion of blood by some blood-feeding parasites. These hematophagous organisms such as malaria parasites, Rhodnius and Schistosoma digest haemoglobin and release high quantities of free heme, which is the non-protein component of haemoglobin. Heme is a prosthetic group consisting of an iron atom contained in the center of a heterocyclic porphyrin ring. Free heme is toxic to cells, so the parasites convert it into an insoluble crystalline form called hemozoin. In malaria parasites, hemozoin is often called malaria pigment.
Human genetic resistance to malaria refers to inherited changes in the DNA of humans which increase resistance to malaria and result in increased survival of individuals with those genetic changes. The existence of these genotypes is likely due to evolutionary pressure exerted by parasites of the genus Plasmodium which cause malaria. Since malaria infects red blood cells, these genetic changes are most common alterations to molecules essential for red blood cell function, such as hemoglobin or other cellular proteins or enzymes of red blood cells. These alterations generally protect red blood cells from invasion by Plasmodium parasites or replication of parasites within the red blood cell.
Russell J. Howard is an Australian-born executive, entrepreneur and scientist. He was a pioneer in the fields of molecular parasitology, especially malaria, and in leading the commercialisation of one of the most important methods used widely today in molecular biology today called “DNA shuffling" or "Molecular breeding", a form of "Directed evolution".
KAHRP is a protein expressed by Plasmodium falciparum infecting erythrocytes. KAHRP is a major component of knobs, feature found on Plasmodium falciparum infected erythrocytes.
Sanaria is a biotechnology company developing vaccines protective against malaria and other infectious diseases as well as related products for use in malaria research. Sanaria's vaccines are based on the use of the sporozoite (SPZ) stage of the malaria parasite, Plasmodium, as an immunogen, and as a platform technology for liver-vectored gene delivery. SPZ are normally introduced into humans by mosquito bite where they migrate to the liver and further develop to liver stages, and eventually back into the blood stream where the parasite infects red blood cells (RBC) and causes malaria. Plasmodium falciparum is the species responsible for more than 95% deaths caused by malaria. The WHO estimates there were 249 million clinical cases and 608,000 deaths in 2022 alone.
Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a family of proteins present on the membrane surface of red blood cells that are infected by the malarial parasite Plasmodium falciparum. PfEMP1 is synthesized during the parasite's blood stage inside the RBC, during which the clinical symptoms of falciparum malaria are manifested. Acting as both an antigen and adhesion protein, it is thought to play a key role in the high level of virulence associated with P. falciparum. It was discovered in 1984 when it was reported that infected RBCs had unusually large-sized cell membrane proteins, and these proteins had antibody-binding (antigenic) properties. An elusive protein, its chemical structure and molecular properties were revealed only after a decade, in 1995. It is now established that there is not one but a large family of PfEMP1 proteins, genetically regulated (encoded) by a group of about 60 genes called var. Each P. falciparum is able to switch on and off specific var genes to produce a functionally different protein, thereby evading the host's immune system. RBCs carrying PfEMP1 on their surface stick to endothelial cells, which facilitates further binding with uninfected RBCs, ultimately helping the parasite to both spread to other RBCs as well as bringing about the fatal symptoms of P. falciparum malaria.

The Plasmodium helical interspersed subtelomeric proteins (PHIST) or ring-infected erythrocyte surface antigens (RESA) are a family of protein domains found in the malaria-causing Plasmodium species. It was initially identified as a short four-helical conserved region in the single-domain export proteins, but the identification of this part associated with a DnaJ domain in P. falciparum RESA has led to its reclassification as the RESA N-terminal domain. This domain has been classified into three subfamilies, PHISTa, PHISTb, and PHISTc.
Reticulocyte binding protein homologs (RHs) are a superfamily of proteins found in Plasmodium responsible for cell invasion. Together with the family of erythrocyte binding-like proteins (EBLs) they make up the two families of invasion proteins universal to Plasmodium. The two families function cooperatively.
References
- 1 2 3 4 5 6 Srivastava A, Gangnard S, Round A, Dechavanne S, Juillerat A, Raynal B, et al. (March 2010). "Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA". Proceedings of the National Academy of Sciences of the United States of America. 107 (11): 4884–9. Bibcode:2010PNAS..107.4884S. doi: 10.1073/pnas.1000951107 . PMC 2841952 . PMID 20194779.
- Lay summary in: "Malaria in pregnant women: Step towards a new vaccine". Science Daily (Press release). March 12, 2010.
- ↑ "CDC-Malaria-Malaria Parasites". Centers for Disease Control and Prevention. 2019-01-28.
- ↑ Perlmann P, Troye-Blomberg M (2000). "Malaria blood-stage infection and its control by the immune system". Folia Biologica. 46 (6): 210–8. PMID 11140853.
- ↑ "Lives at Risk: Malaria in Pregnancy". WHO. Archived from the original on May 7, 2003. Retrieved March 30, 2011.
- ↑ Duffy PE, Fried M (2005). "Malaria in the Pregnant Woman". Malaria: Drugs, Disease and Post-genomic Biology. Current Topics in Microbiology and Immunology. Vol. 295. pp. 169–200. doi:10.1007/3-540-29088-5_7. ISBN 978-3-540-25363-1. PMID 16265891.
- 1 2 "Roll Back Malaria: Malaria in Pregnancy". WHO. Archived from the original on 6 August 2006. Retrieved 18 April 2011.
- ↑ Doritchamou J, Teo A, Fried M, Duffy PE (2019). "Malaria in pregnancy: the relevance of animal models for vaccine development". Lab Animal. 46 (10): 388–398. doi:10.1038/laban.1349. PMC 6771290 . PMID 28984865.
- ↑ Doolan DL, Dobaño C, Baird JK (January 2009). "Acquired immunity to malaria". Clinical Microbiology Reviews. 22 (1): 13–36, Table of Contents. doi:10.1128/CMR.00025-08. PMC 2620631 . PMID 19136431.
- ↑ Matteelli A, Caligaris S, Castelli F, Carosi G (October 1997). "The placenta and malaria". Annals of Tropical Medicine and Parasitology. 91 (7): 803–10. doi:10.1080/00034989760563. PMID 9625937.
- ↑ White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM (2014). "Malaria". The Lancet. 383 (9918): 723–735. doi:10.1016/s0140-6736(13)60024-0. PMID 23953767. S2CID 208794141.
- 1 2 3 4 Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD (February 2007). "Epidemiology and burden of malaria in pregnancy". The Lancet. Infectious Diseases. 7 (2): 93–104. doi:10.1016/S1473-3099(07)70021-X. PMID 17251080.
- ↑ "Burden of Malaria in Pregnancy in Latin America Not Known". Centers for Disease Control and Prevention. Retrieved April 14, 2011.
- ↑ Kumar H, Tolia NH (September 2019). "Getting in: The structural biology of malaria invasion". PLOS Pathogens. 15 (9): e1007943. doi: 10.1371/journal.ppat.1007943 . PMC 6728024 . PMID 31487334.
- ↑ Schantz-Dunn J, Nour NM (2009). "Malaria and pregnancy: a global health perspective". Reviews in Obstetrics & Gynecology. 2 (3): 186–92. PMC 2760896 . PMID 19826576.
- ↑ "WHO | Information for travellers". WHO. Archived from the original on December 25, 2009. Retrieved 2020-08-04.
- 1 2 Hviid L, Salanti A (2007). "VAR2CSA and protective immunity against pregnancy-associated Plasmodium falciparum malaria". Parasitology. 134 (Pt 13): 1871–6. doi:10.1017/S0031182007000121. PMID 17958922. S2CID 7706073.
- ↑ Zakama AK, Gaw SL (September 2019). "Malaria in Pregnancy: What the Obstetric Provider in Nonendemic Areas Needs to Know". Obstetrical & Gynecological Survey. 74 (9): 546–556. doi:10.1097/OGX.0000000000000704. PMC 7560991 . PMID 31830300.
- ↑ Gamain B, Smith JD, Viebig NK, Gysin J, Scherf A (March 2007). "Pregnancy-associated malaria: parasite binding, natural immunity and vaccine development". International Journal for Parasitology. 37 (3–4): 273–83. doi:10.1016/j.ijpara.2006.11.011. PMID 17224156.
- 1 2 Kwenti TE (2018). "Malaria and HIV coinfection in sub-Saharan Africa: prevalence, impact, and treatment strategies". Research and Reports in Tropical Medicine. 9: 123–136. doi: 10.2147/rrtm.s154501 . PMC 6067790 . PMID 30100779.
- ↑ Frischknecht F, Fackler OT (July 2016). "Experimental systems for studying Plasmodium/HIV coinfection". FEBS Letters. 590 (13): 2000–13. doi: 10.1002/1873-3468.12151 . PMID 27009943.
- ↑ Fried M, Duffy PE (June 1996). "Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta". Science. 272 (5267). New York, N.Y.: 1502–4. Bibcode:1996Sci...272.1502F. doi:10.1126/science.272.5267.1502. PMID 8633247. S2CID 43040825.
- 1 2 3 Nielsen MA, Pinto VV, Resende M, Dahlbäck M, Ditlev SB, Theander TG, Salanti A (June 2009). "Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA". Infection and Immunity. 77 (6): 2482–7. doi:10.1128/IAI.00159-09. PMC 2687338 . PMID 19307213.
- ↑ David PH, Hommel M, Miller LH, Udeinya IJ, Oligino LD (August 1983). "Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes". Proceedings of the National Academy of Sciences of the United States of America. 80 (16): 5075–9. Bibcode:1983PNAS...80.5075D. doi: 10.1073/pnas.80.16.5075 . PMC 384191 . PMID 6348780.
- 1 2 3 Resende M, Ditlev SB, Nielsen MA, Bodevin S, Bruun S, Pinto VV, Clausen H, Turner L, Theander TG, Salanti A, Dahlbäck M (September 2009). "Chondroitin sulphate A (CSA)-binding of single recombinant Duffy-binding-like domains is not restricted to Plasmodium falciparum Erythrocyte Membrane Protein 1 expressed by CSA-binding parasites". International Journal for Parasitology. 39 (11): 1195–204. doi:10.1016/j.ijpara.2009.02.022. PMID 19324047.
- ↑ Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG (July 2003). "Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria". Molecular Microbiology. 49 (1): 179–91. doi: 10.1046/j.1365-2958.2003.03570.x . PMID 12823820. S2CID 38384882.
- ↑ Viebig NK, Gamain B, Scheidig C, Lépolard C, Przyborski J, Lanzer M, Gysin J, Scherf A (August 2005). "A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A". EMBO Reports. 6 (8): 775–81. doi:10.1038/sj.embor.7400466. PMC 1369142 . PMID 16025132.
- ↑ Gamain B, Trimnell AR, Scheidig C, Scherf A, Miller LH, Smith JD (March 2005). "Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites". The Journal of Infectious Diseases. 191 (6): 1010–3. doi: 10.1086/428137 . PMID 15717280.
- ↑ Bancells C, Deitsch KW (November 2013). "A molecular switch in the efficiency of translation reinitiation controls expression of var2csa, a gene implicated in pregnancy-associated malaria". Molecular Microbiology. 90 (3): 472–88. doi:10.1111/mmi.12379. PMC 3938558 . PMID 23980802.
- ↑ Duffy MF, Caragounis A, Noviyanti R, Kyriacou HM, Choong EK, Boysen K, et al. (August 2006). "Transcribed var genes associated with placental malaria in Malawian women". Infection and Immunity. 74 (8): 4875–83. doi:10.1128/IAI.01978-05. PMC 1539630 . PMID 16861676.
- ↑ Chan S, Frasch A, Mandava CS, Ch'ng JH, Quintana MD, Vesterlund M, et al. (May 2017). "Regulation of PfEMP1-VAR2CSA translation by a Plasmodium translation-enhancing factor". Nature Microbiology. 2 (7): 17068. doi:10.1038/nmicrobiol.2017.68. PMID 28481333. S2CID 2276480.
- ↑ Thompson JM, Eick SM, Dailey C, Dale AP, Mehta M, Nair A, et al. (June 2020). "Relationship Between Pregnancy-Associated Malaria and Adverse Pregnancy Outcomes: a Systematic Review and Meta-Analysis". Journal of Tropical Pediatrics. 66 (3): 327–338. doi: 10.1093/tropej/fmz068 . PMID 31598714.
- ↑ Cutts JC, Agius PA, Powell R, Moore K, Draper B, Simpson JA, Fowkes FJ (January 2020). "Pregnancy-specific malarial immunity and risk of malaria in pregnancy and adverse birth outcomes: a systematic review". BMC Medicine. 18 (1): 14. doi: 10.1186/s12916-019-1467-6 . PMC 6964062 . PMID 31941488.
- 1 2 3 4 5 6 7 8 9 10 World Malaria Report 2019. World Health Organization. 2019. ISBN 978-92-4-156572-1. OCLC 1156338614.
- ↑ Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T (July 2015). "Daily oral iron supplementation during pregnancy". The Cochrane Database of Systematic Reviews. 2015 (7): CD004736. doi:10.1002/14651858.CD004736.pub5. PMC 4233117 . PMID 26198451.
- ↑ Park S, Nixon CE, Miller O, Choi NK, Kurtis JD, Friedman JF, Michelow IC (July 2020). "Impact of Malaria in Pregnancy on Risk of Malaria in Young Children: Systematic Review and Meta-Analyses". The Journal of Infectious Diseases. 222 (4): 538–550. doi:10.1093/infdis/jiaa139. PMC 7377293 . PMID 32219317.
- 1 2 Cot M, Deloron P (2003). "Malaria prevention strategies". British Medical Bulletin. 67 (1): 137–48. doi: 10.1093/bmb/ldg003 . PMID 14711760.
- ↑ CDC-Centers for Disease Control and Prevention (2019). "CDC - Malaria - Travelers - Choosing a Drug to Prevent Malaria". www.cdc.gov. Retrieved 2020-07-31.
- 1 2 Moya-Alvarez V, Abellana R, Cot M (July 2014). "Pregnancy-associated malaria and malaria in infants: an old problem with present consequences". Malaria Journal. 13 (1): 271. doi: 10.1186/1475-2875-13-271 . PMC 4113781 . PMID 25015559.
- ↑ Agboghoroma CO (2014). "Current management and prevention of malaria in pregnancy: a review". West African Journal of Medicine. 33 (2): 91–9. PMID 25236824.
- ↑ "Fact sheet about Malaria". www.who.int. Retrieved 2020-08-02.
- ↑ Gueneuc A, Deloron P, Bertin GI (January 2017). "Usefulness of a biomarker to identify placental dysfunction in the context of malaria". Malaria Journal. 16 (1): 11. doi: 10.1186/s12936-016-1664-0 . PMC 5209802 . PMID 28049536.
- ↑ ter Kuile FO, van Eijk AM, Filler SJ (June 2007). "Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review". JAMA. 297 (23): 2603–16. doi:10.1001/jama.297.23.2603. PMID 17579229.
- ↑ "The use of non-pharmaceutical forms of Artemisia". www.who.int. Archived from the original on June 7, 2020. Retrieved 2020-08-04.
- 1 2 World Health Organization (13 August 2015). Guidelines for the treatment of malaria (Third ed.). Geneva. ISBN 978-92-4-154912-7. OCLC 908628497.
{{cite book}}: CS1 maint: location missing publisher (link) - 1 2 Bauserman M, Conroy AL, North K, Patterson J, Bose C, Meshnick S (August 2019). "An overview of malaria in pregnancy". Seminars in Perinatology. 43 (5): 282–290. doi:10.1053/j.semperi.2019.03.018. hdl: 1805/22076 . PMC 7895297 . PMID 30979598.
- ↑ "Malaria - Diagnosis & Treatment (United States) - Treatment (U.S.) - Guidelines for Clinicians (Part 3)". www.cdc.gov. CDC-Centers for Disease Control and Prevention. 2019-03-27. Retrieved 2020-08-02.
- ↑ Worldwide Antimalarial Resistance Network (WWARN) (2016-01-28). "Malaria in Pregnancy Consortium". Worldwide Antimalarial Resistance Network. Retrieved 2020-08-04.
- ↑ "CDC - Malaria - About Malaria - Where Malaria Occurs". www.cdc.gov. CDC-Centers for Disease Control and Prevention. 2020. Retrieved 2020-07-31.
- 1 2 World Health Organization. "Malaria in pregnant women". WHO. Retrieved 2020-07-31.
- ↑ United Kingdom National Health Service (2018). "Malaria - Causes". nhs.uk. Retrieved 2020-07-31.
- ↑ Andersen P, Nielsen MA, Resende M, Rask TS, Dahlbäck M, Theander T, Lund O, Salanti A (February 2008). "Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA". PLOS Pathogens. 4 (2): e42. doi: 10.1371/journal.ppat.0040042 . PMC 2242842 . PMID 18282103.
- ↑ Avril M, Gamain B, Lépolard C, Viaud N, Scherf A, Gysin J (2006). "Characterization of anti-var2CSA-PfEMP1 cytoadhesion inhibitory mouse monoclonal antibodies". Microbes and Infection. 8 (14–15): 2863–71. doi: 10.1016/j.micinf.2006.09.005 . PMID 17095277.
- ↑ Fernandez P, Viebig NK, Dechavanne S, Lépolard C, Gysin J, Scherf A, Gamain B (September 2008). "Var2CSA DBL6-epsilon domain expressed in HEK293 induces limited cross-reactive and blocking antibodies to CSA binding parasites". Malaria Journal. 7: 170. doi: 10.1186/1475-2875-7-170 . PMC 2543044 . PMID 18771584.
- 1 2 "G6PD gene". Genetics Home Reference. Retrieved 2020-08-05.
- 1 2 Brummaier T, Gilder ME, Gornsawun G, Chu CS, Bancone G, Pimanpanarak M, et al. (January 2020). "Vivax malaria in pregnancy and lactation: a long way to health equity". Malaria Journal. 19 (1): 40. doi: 10.1186/s12936-020-3123-1 . PMC 6977346 . PMID 31969155.
- 1 2 Mordmüller B, Sulyok M, Egger-Adam D, Resende M, de Jongh WA, Jensen MH, et al. (October 2019). "First-in-human, Randomized, Double-blind Clinical Trial of Differentially Adjuvanted PAMVAC, A Vaccine Candidate to Prevent Pregnancy-associated Malaria". Clinical Infectious Diseases. 69 (9): 1509–1516. doi:10.1093/cid/ciy1140. PMC 6792113 . PMID 30629148.
- ↑ GEN (2019-01-10). "Pregnancy-Associated Malaria Vaccine Passes First Human Trial". GEN - Genetic Engineering and Biotechnology News. Retrieved 2020-08-02.
- ↑ Chêne A, Gangnard S, Guadall A, Ginisty H, Leroy O, Havelange N, et al. (April 2019). "Preclinical immunogenicity and safety of the cGMP-grade placental malaria vaccine PRIMVAC". eBioMedicine. 42: 145–156. doi:10.1016/j.ebiom.2019.03.010. PMC 6491931 . PMID 30885725.