Related Research Articles

Anticoagulants, commonly known as blood thinners, are chemical substances that prevent or reduce coagulation of blood, prolonging the clotting time. Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where they help keep the bite area unclotted long enough for the animal to obtain some blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, heart–lung machines, and dialysis equipment. One of the first anticoagulants, warfarin, was initially approved as a rodenticide.
Thrombosis is the formation of a blood clot inside a blood vessel, obstructing the flow of blood through the circulatory system. When a blood vessel is injured, the body uses platelets (thrombocytes) and fibrin to form a blood clot to prevent blood loss. Even when a blood vessel is not injured, blood clots may form in the body under certain conditions. A clot, or a piece of the clot, that breaks free and begins to travel around the body is known as an embolus.
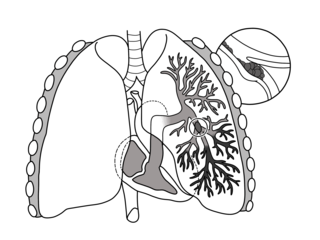
Pulmonary embolism (PE) is a blockage of an artery in the lungs by a substance that has moved from elsewhere in the body through the bloodstream (embolism). Symptoms of a PE may include shortness of breath, chest pain particularly upon breathing in, and coughing up blood. Symptoms of a blood clot in the leg may also be present, such as a red, warm, swollen, and painful leg. Signs of a PE include low blood oxygen levels, rapid breathing, rapid heart rate, and sometimes a mild fever. Severe cases can lead to passing out, abnormally low blood pressure, obstructive shock, and sudden death.

Venous thrombosis is the blockage of a vein caused by a thrombus. A common form of venous thrombosis is deep vein thrombosis (DVT), when a blood clot forms in the deep veins. If a thrombus breaks off (embolizes) and flows to the lungs to lodge there, it becomes a pulmonary embolism (PE), a blood clot in the lungs. The conditions of DVT only, DVT with PE, and PE only, are all captured by the term venous thromboembolism (VTE).
Antiphospholipid syndrome, or antiphospholipid antibody syndrome, is an autoimmune, hypercoagulable state caused by antiphospholipid antibodies. APS can lead to blood clots (thrombosis) in both arteries and veins, pregnancy-related complications, and other symptoms like low platelets, kidney disease, heart disease, and rash. Although the exact etiology of APS is still not clear, genetics is believed to play a key role in the development of the disease. Diagnosis is made based on symptoms and testing, but sometimes research criteria is used to aid in diagnosis. The research criteria for definite APS requires one clinical event and two positive blood test results spaced at least three months apart that detect lupus anticoagulant, anti-apolipoprotein antibodies, and/or anti-cardiolipin antibodies.
Factor V Leiden is a variant of human factor V, which causes an increase in blood clotting (hypercoagulability). Due to this mutation, protein C, an anticoagulant protein that normally inhibits the pro-clotting activity of factor V, is not able to bind normally to factor V, leading to a hypercoagulable state, i.e., an increased tendency for the patient to form abnormal and potentially harmful blood clots. Factor V Leiden is the most common hereditary hypercoagulability disorder amongst ethnic Europeans. It is named after the Dutch city of Leiden, where it was first identified in 1994 by Rogier Maria Bertina under the direction of Pieter Hendrick Reitsma. Despite the increased risk of venous thromboembolisms, people with one copy of this gene have not been found to have shorter lives than the general population. It is an autosomal dominant genetic disorder with incomplete penetrance.

Deep vein thrombosis (DVT) is a type of venous thrombosis involving the formation of a blood clot in a deep vein, most commonly in the legs or pelvis. A minority of DVTs occur in the arms. Symptoms can include pain, swelling, redness, and enlarged veins in the affected area, but some DVTs have no symptoms. The most common life-threatening concern with DVT is the potential for a clot to embolize, travel as an embolus through the right side of the heart, and become lodged in a pulmonary artery that supplies blood to the lungs. This is called a pulmonary embolism (PE). DVT and PE comprise the cardiovascular disease of venous thromboembolism (VTE). About two-thirds of VTE manifests as DVT only, with one-third manifesting as PE with or without DVT. The most frequent long-term DVT complication is post-thrombotic syndrome, which can cause pain, swelling, a sensation of heaviness, itching, and in severe cases, ulcers. Recurrent VTE occurs in about 30% of those in the ten years following an initial VTE.
Low-molecular-weight heparin (LMWH) is a class of anticoagulant medications. They are used in the prevention of blood clots and treatment of venous thromboembolism and in the treatment of myocardial infarction.

Heparin-induced thrombocytopenia (HIT) is the development of thrombocytopenia, due to the administration of various forms of heparin, an anticoagulant. HIT predisposes to thrombosis. When thrombosis is identified the condition is called heparin-induced thrombocytopenia and thrombosis (HITT). HIT is caused by the formation of abnormal antibodies that activate platelets, which release microparticles that activate thrombin, leading to thrombosis. If someone receiving heparin develops new or worsening thrombosis, or if the platelet count falls, HIT can be confirmed with specific blood tests.

Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis. Such abnormalities can be identified in 50% of people who have an episode of thrombosis that was not provoked by other causes. A significant proportion of the population has a detectable thrombophilic abnormality, but most of these develop thrombosis only in the presence of an additional risk factor.

Renal vein thrombosis (RVT) is the formation of a clot in the vein that drains blood from the kidneys, ultimately leading to a reduction in the drainage of one or both kidneys and the possible migration of the clot to other parts of the body. First described by German pathologist Friedrich Daniel von Recklinghausen in 1861, RVT most commonly affects two subpopulations: newly born infants with blood clotting abnormalities or dehydration and adults with nephrotic syndrome.

Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication used to treat and prevent blood clots. Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery. It is taken by mouth.

Activated protein C resistance (APCR) is a hypercoagulability characterized by a lack of a response to activated protein C (APC), which normally helps prevent blood from clotting excessively. This results in an increased risk of venous thrombosis, which resulting in medical conditions such as deep vein thrombosis and pulmonary embolism. The most common cause of hereditary APC resistance is factor V Leiden mutation.

Protein C deficiency is a rare genetic trait that predisposes to thrombotic disease. It was first described in 1981. The disease belongs to a group of genetic disorders known as thrombophilias. Protein C deficiency is associated with an increased incidence of venous thromboembolism, whereas no association with arterial thrombotic disease has been found.
Antithrombin III deficiency is a deficiency of antithrombin III. This deficiency may be inherited or acquired. It is a rare hereditary disorder that generally comes to light when a patient suffers recurrent venous thrombosis and pulmonary embolism, and repetitive intrauterine fetal death (IUFD). Hereditary antithrombin deficiency results in a state of increased coagulation which may lead to venous thrombosis. Inheritance is usually autosomal dominant, though a few recessive cases have been noted. The disorder was first described by Egeberg in 1965. The causes of acquired antithrombin deficiency are easier to find than the hereditary deficiency.

Tinzaparin is an antithrombotic drug in the heparin group. It is a low molecular weight heparin (LMWH) marketed as Innohep worldwide. It has been approved by the U.S. Food and Drug Administration (FDA) for once daily treatment and prophylaxis of deep vein thrombosis (DVT) and pulmonary embolism (PE).
Blood clots are a relatively common occurrence in the general population and are seen in approximately 1-2% of the population by age 60. Typically, blood clots develop in the deep veins of the lower extremities, deep vein thrombosis (DVT) or as a blood clot in the lung, pulmonary embolism. A very small number of people who develop blood clots have a more serious and often life-threatening condition, known as thrombotic storm (TS). TS is characterized by the development of more than one blood clot in a short period of time. These clots often occur in multiple and sometimes unusual locations in the body and are often difficult to treat. TS may be associated with an existing condition or situation that predisposes a person to blood clots, such as injury, infection, or pregnancy. In many cases, a risk assessment will identify interventions that will prevent the formation of blood clots.
Prothrombin G20210A is a genetic condition that increases the risk of blood clots including from deep vein thrombosis, and of pulmonary embolism. One copy of the mutation increases the risk of a blood clot from 1 in 1,000 per year to 2.5 in 1,000. Two copies increases the risk to up to 20 in 1,000 per year. Most people never develop a blood clot in their lifetimes.
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways. DTIs have undergone rapid development since the 90's. With technological advances in genetic engineering the production of recombinant hirudin was made possible which opened the door to this new group of drugs. Before the use of DTIs the therapy and prophylaxis for anticoagulation had stayed the same for over 50 years with the use of heparin derivatives and warfarin which have some well known disadvantages. DTIs are still under development, but the research focus has shifted towards factor Xa inhibitors, or even dual thrombin and fXa inhibitors that have a broader mechanism of action by both inhibiting factor IIa (thrombin) and Xa. A recent review of patents and literature on thrombin inhibitors has demonstrated that the development of allosteric and multi-mechanism inhibitors might lead the way to a safer anticoagulant.

Thrombosis prevention or thromboprophylaxis is medical treatment to prevent the development of thrombosis in those considered at risk for developing thrombosis. Some people are at a higher risk for the formation of blood clots than others, such as those with cancer undergoing a surgical procedure. Prevention measures or interventions are usually begun after surgery as the associated immobility will increase a person's risk.
References
- 1 2 3 Page 264 in: Gresele, Paolo (2008). Platelets in hematologic and cardiovascular disorders: a clinical handbook. Cambridge, UK: Cambridge University Press. ISBN 978-0-521-88115-9.
- 1 2 3 4 5 6 7 8 Hypercoagulability during Pregnancy Lab Lines. A publication of the Department of Pathology and Laboratory Medicine at the University of Cincinnati. September/October 2002 Volume 8, Issue 5
- ↑ de Boer K, ten Cate JW, Sturk A, Borm JJ, Treffers PE (1989). "Enhanced thrombin generation in normal and hypertensive pregnancy". Am J Obstet Gynecol. 160 (1): 95–100. doi:10.1016/0002-9378(89)90096-3. PMID 2521425.
- ↑ "Venous Thromboembolism (Blood Clots) and Pregnancy". Centers for Disease Control and Prevention. 20 August 2020. Retrieved 24 October 2020.
- 1 2 Abdul Sultan, A.; West, J.; Tata, L. J.; Fleming, K. M.; Nelson-Piercy, C.; Grainge, M. J. (2013). "Risk of first venous thromboembolism in pregnant women in hospital: Population based cohort study from England". BMJ. 347: f6099. doi:10.1136/bmj.f6099. PMC 3898207 . PMID 24201164.
- ↑ Eichinger, S.; Evers, J. L. H.; Glasier, A.; La Vecchia, C.; Martinelli, I.; Skouby, S.; Somigliana, E.; Baird, D. T.; Benagiano, G.; Crosignani, P. G.; Gianaroli, L.; Negri, E.; Volpe, A.; Glasier, A.; Crosignani, P. G. (2013). "Venous thromboembolism in women: A specific reproductive health risk". Human Reproduction Update. 19 (5): 471–482. doi: 10.1093/humupd/dmt028 . PMID 23825156.
- 1 2 de Vries JI, van Pampus MG, Hague WM, Bezemer PD, Joosten JH, FRUIT Investigators (2012). "Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: the FRUIT-RCT". J. Thromb. Haemost. 10 (1): 64–72. doi: 10.1111/j.1538-7836.2011.04553.x . PMID 22118560.
- ↑ McNamee, Kelly; Dawood, Feroza; Farquharson, Roy (1 August 2012). "Recurrent miscarriage and thrombophilia". Current Opinion in Obstetrics and Gynecology. 24 (4): 229–234. doi:10.1097/GCO.0b013e32835585dc. PMID 22729089.
- 1 2 3 4 5 6 7 8 9 "Hemostasrubbningar inom obstetrik och gynekologi" (Disorders of hemostasis in obstetrics and gynecology), from ARG (work and reference group) from SFOG (Swedish association of obstetrics and gynecology). Intro available at . Updated 2012.
- ↑ Giannubilo, SR; Tranquilli, AL (2012). "Anticoagulant therapy during pregnancy for maternal and fetal acquired and inherited thrombophilia". Current Medicinal Chemistry. 19 (27): 4562–71. doi:10.2174/092986712803306466. PMID 22876895.
- ↑ Sathienkijkanchai A, Wasant P (2005). "Fetal warfarin syndrome". J Med Assoc Thai. 88 (Suppl 8): S246–50. PMID 16856447.
- ↑ Schaefer C, Hannemann D, Meister R, Eléfant E, Paulus W, Vial T, Reuvers M, Robert-Gnansia E, Arnon J, De Santis M, Clementi M, Rodriguez-Pinilla E, Dolivo A, Merlob P (2006). "Vitamin K antagonists and pregnancy outcome. A multi-centre prospective study". Thromb Haemost. 95 (6): 949–57. doi:10.1160/TH06-02-0108. PMID 16732373. S2CID 33278534.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Archived 12 June 2010 at the Wayback Machine Therapeutic anticoagulation in pregnancy. Norfolk and Norwich University Hospital (NHS Trust). Reference number CA3017. 9 June 2006 [review June 2009]
- ↑ Couto E, Nomura ML, Barini R, Pinto e Silva JL (2005). "Pregnancy-associated venous thromboembolism in combined heterozygous factor V Leiden and prothrombin G20210A mutations". Sao Paulo Med J. 123 (6): 286–8. doi: 10.1590/S1516-31802005000600007 . PMID 16444389.
- ↑ De Jong, P. G.; Goddijn, M.; Middeldorp, S. (2013). "Antithrombotic therapy for pregnancy loss". Human Reproduction Update. 19 (6): 656–673. doi: 10.1093/humupd/dmt019 . PMID 23766357.
- ↑ Shaul WL, Emery H, Hall JG (1975). "Chondrodysplasia punctata and maternal warfarin use during pregnancy". Am J Dis Child. 129 (3): 360–2. doi:10.1001/archpedi.1975.02120400060014. PMID 1121966.
- ↑ James AH, Grotegut CA, Brancazio LR, Brown H (2007). "Thromboembolism in pregnancy: recurrence and its prevention". Semin Perinatol. 31 (3): 167–75. doi:10.1053/j.semperi.2007.03.002. PMID 17531898.
- ↑ Kim BJ, An SJ, Shim SS, Jun JK, Yoon BH, Syn HC, Park JS (2006). "Pregnancy outcomes in women with mechanical heart valves". J Reprod Med. 51 (8): 649–54. PMID 16967636.
- ↑ Iturbe-Alessio I, Fonseca MC, Mutchinik O, Santos MA, Zajarías A, Salazar E (1986). "Risks of anticoagulant therapy in pregnant women with artificial heart valves". N Engl J Med. 315 (22): 1390–3. doi:10.1056/NEJM198611273152205. PMID 3773964.
- ↑ Salazar E, Izaguirre R, Verdejo J, Mutchinick O (1996). "Failure of adjusted doses of subcutaneous heparin to prevent thromboembolic phenomena in pregnant patients with mechanical cardiac valve prostheses". J Am Coll Cardiol. 27 (7): 1698–703. doi: 10.1016/0735-1097(96)00072-1 . PMID 8636556.
- ↑ Ginsberg JS, Chan WS, Bates SM, Kaatz S (2003). "Anticoagulation of pregnant women with mechanical heart valves" (PDF). Arch Intern Med. 163 (6): 694–8. doi: 10.1001/archinte.163.6.694 . PMID 12639202.