Amniocentesis is a medical procedure used primarily in the prenatal diagnosis of genetic conditions. It has other uses such as in the assessment of infection and fetal lung maturity. Prenatal diagnostic testing, which includes amniocentesis, is necessary to conclusively diagnose the majority of genetic disorders, with amniocentesis being the gold-standard procedure after 15 weeks' gestation.

Preterm birth, also known as premature birth, is the birth of a baby at fewer than 37 weeks gestational age, as opposed to full-term delivery at approximately 40 weeks. Extreme preterm is less than 28 weeks, very early preterm birth is between 28 and 32 weeks, early preterm birth occurs between 32 and 34 weeks, late preterm birth is between 34 and 36 weeks' gestation. These babies are also known as premature babies or colloquially preemies or premmies. Symptoms of preterm labor include uterine contractions which occur more often than every ten minutes and/or the leaking of fluid from the vagina before 37 weeks. Premature infants are at greater risk for cerebral palsy, delays in development, hearing problems and problems with their vision. The earlier a baby is born, the greater these risks will be.

Selective reduction is the practice of reducing the number of fetuses in a multiple pregnancy, such as quadruplets, to a twin or singleton pregnancy. The procedure is also called multifetal pregnancy reduction. The procedure is most commonly done to reduce the number of fetuses in a multiple pregnancy to a safe number, when the multiple pregnancy is the result of use of assisted reproductive technology; outcomes for both the mother and the babies are generally worse the higher the number of fetuses. The procedure is also used in multiple pregnancies when one of the fetuses has a serious and incurable disease, or in the case where one of the fetuses is outside the uterus, in which case it is called selective termination.

External cephalic version (ECV) is a process by which a breech baby can sometimes be turned from buttocks or foot first to head first. It is a manual procedure that is recommended by national guidelines for breech presentation of a pregnancy with a single baby, in order to enable vaginal delivery. It is usually performed late in pregnancy, that is, after 36 gestational weeks, preferably 37 weeks, and can even be performed in early labour.
Oligohydramnios is a medical condition in pregnancy characterized by a deficiency of amniotic fluid, the fluid that surrounds the fetus in the abdomen, in the amniotic sac. It is typically diagnosed by ultrasound when the amniotic fluid index (AFI) measures less than 5 cm or when the single deepest pocket (SDP) of amniotic fluid measures less than 2 cm. Amniotic fluid is necessary to allow for normal fetal movement, lung development, and cushioning from uterine compression. Low amniotic fluid can be attributed to a maternal, fetal, placental or idiopathic cause and can result in poor fetal outcomes including death. The prognosis of the fetus is dependent on the etiology, gestational age at diagnosis, and the severity of the oligohydramnios.

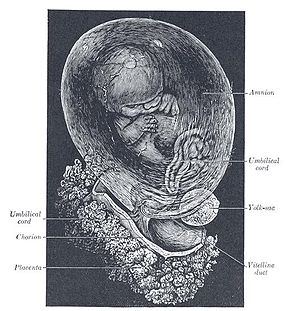
The amniotic fluid is the protective liquid contained by the amniotic sac of a gravid amniote. This fluid serves as a cushion for the growing fetus, but also serves to facilitate the exchange of nutrients, water, and biochemical products between mother and fetus.
Labor induction is the process or treatment that stimulates childbirth and delivery. Inducing (starting) labor can be accomplished with pharmaceutical or non-pharmaceutical methods. In Western countries, it is estimated that one-quarter of pregnant women have their labor medically induced with drug treatment. Inductions are most often performed either with prostaglandin drug treatment alone, or with a combination of prostaglandin and intravenous oxytocin treatment.
Rupture of membranes (ROM) or amniorrhexis is a term used during pregnancy to describe a rupture of the amniotic sac. Normally, it occurs spontaneously at full term either during or at the beginning of labor. Rupture of the membranes is known colloquially as "breaking [one's] water," especially when induced rather than spontaneous, or as one's "water breaking". A premature rupture of membranes (PROM) is a rupture of the amnion that occurs at full term and prior to the onset of labor. In cases of PROM, options include expectant management without intervention, or interventions such as oxytocin or other methods of labor induction, and both are usually accompanied by close monitoring of maternal and fetal health. Preterm premature rupture of membranes (PPROM) is when water breaks both before the onset of labor and before the pregnancy's 37 week gestation. In the United States, more than 120,000 pregnancies per year are affected by a premature rupture of membranes, which is the cause of about one third of preterm deliveries.
Fetal fibronectin (fFN) is a fibronectin protein produced by fetal cells. It is found at the interface of the chorion and the decidua. Fetal fibronectin is found normally in vaginal fluid in early pregnancy prior to 22 weeks due to normal growth and development of tissues at the junction of the uterus and amniotic sac. It may also be found in vaginal fluid after 36 weeks as labor approaches. However, fFN should not be detected between 22 and 36 weeks.

Complications of pregnancy are health problems that are related to, or arise during pregnancy. Complications that occur primarily during childbirth are termed obstetric labor complications, and problems that occur primarily after childbirth are termed puerperal disorders. While some complications improve or are fully resolved after pregnancy, some may lead to lasting effects, morbidity, or in the most severe cases, maternal or fetal mortality.
Antenatal steroids, also known as antenatal corticosteroids, are medications administered to pregnant women expecting a preterm birth. When administered, these steroids accelerate the maturation of the fetus' lungs, which reduces the likelihood of infant respiratory distress syndrome and infant mortality. The effectiveness of this corticosteroid treatment on humans was first demonstrated in 1972 by Sir Graham Liggins and Ross Howie, during a randomized control trial using betamethasone.

Chorioamnionitis, also known as intra-amniotic infection (IAI), is inflammation of the fetal membranes, usually due to bacterial infection. In 2015, a National Institute of Child Health and Human Development Workshop expert panel recommended use of the term "triple I" to address the heterogeneity of this disorder. The term triple I refers to intrauterine infection or inflammation or both and is defined by strict diagnostic criteria, but this terminology has not been commonly adopted although the criteria are used.
Postterm pregnancy is when a woman has not yet delivered her baby after 42 weeks of gestation, two weeks beyond the typical 40-week duration of pregnancy. Postmature births carry risks for both the mother and the baby, including fetal malnutrition, meconium aspiration syndrome, and stillbirths. After the 42nd week of gestation, the placenta, which supplies the baby with nutrients and oxygen from the mother, starts aging and will eventually fail. Postterm pregnancy is a reason to induce labor.

Velamentous cord insertion is a complication of pregnancy where the umbilical cord is inserted in the fetal membranes. It is a major cause of antepartum hemorrhage that leads to loss of fetal blood and associated with high perinatal mortality. In normal pregnancies, the umbilical cord inserts into the middle of the placental mass and is completely encased by the amniotic sac. The vessels are hence normally protected by Wharton's jelly, which prevents rupture during pregnancy and labor. In velamentous cord insertion, the vessels of the umbilical cord are improperly inserted in the chorioamniotic membrane, and hence the vessels traverse between the amnion and the chorion towards the placenta. Without Wharton's jelly protecting the vessels, the exposed vessels are susceptible to compression and rupture.
Placental alpha microglobulin-1 (PAMG-1) is a human protein that was first isolated in 1975 from amniotic fluid. PAMG-1 is an important biomarker for the detection of premature rupture of fetal membrane (PROM) The high concentration of PAMG-1 in amniotic fluid means it can be used to detect if this fluid is present in the cervico-vaginal discharge of pregnant women; the presence of PAMG-1 in the discharge suggests that amniotic fluid is present, and therefore suggests that PROM has occurred. PAMG-1 was originally referred to as specific alpha-1 globulin of placenta.
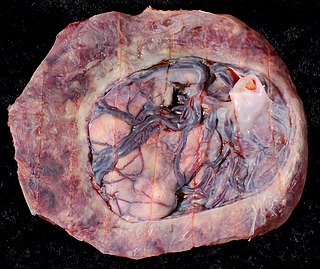
Circumvallate placenta is a rare condition affecting about 1-2% of pregnancies, in which the amnion and chorion fetal membranes essentially "double back" on the fetal side around the edges of the placenta. After delivery, a circumvallate placenta has a thick ring of membranes on its fetal surface. Circumvallate placenta is a placental morphological abnormality associated with increased fetal morbidity and mortality due to the restricted availability of nutrients and oxygen to the developing fetus.
Amnioinfusion is a method in which isotonic fluid is instilled into the uterine cavity.
Artificial rupture of membranes (AROM), also known as an amniotomy, is performed by a midwife or obstetrician and was once thought to be an effective means to induce or accelerate labor. The membranes can be ruptured using a specialized tool, such as an amnihook or amnicot, or they may be ruptured by the proceduralist's finger. The different techniques for artificial rupture of membranes have not been extensively compared in the literature. In one study comparing amnihook versus amnicot for artificial rupture of membranes, use of an amnicot was associated with fewer neonatal scalp lacerations.

A high-risk pregnancy is a pregnancy where the mother or the fetus has an increased risk of adverse outcomes compared to uncomplicated pregnancies. No concrete guidelines currently exist for distinguishing “high-risk” pregnancies from “low-risk” pregnancies; however, there are certain studied conditions that have been shown to put the mother or fetus at a higher risk of poor outcomes. These conditions can be classified into three main categories: health problems in the mother that occur before she becomes pregnant, health problems in the mother that occur during pregnancy, and certain health conditions with the fetus.

Neonatal infections are infections of the neonate (newborn) acquired during prenatal development or within the first four weeks of life. Neonatal infections may be contracted by mother to child transmission, in the birth canal during childbirth, or after birth. Neonatal infections may present soon after delivery, or take several weeks to show symptoms. Some neonatal infections such as HIV, hepatitis B, and malaria do not become apparent until much later. Signs and symptoms of infection may include respiratory distress, temperature instability, irritability, poor feeding, failure to thrive, persistent crying and skin rashes.