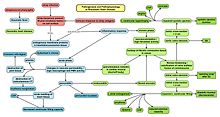
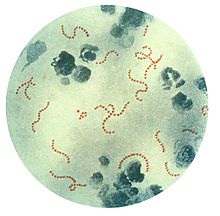
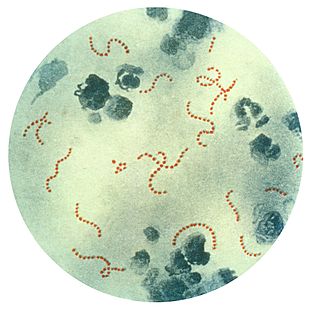
Streptococcus is a genus of gram-positive coccus or spherical bacteria that belongs to the family Streptococcaceae, within the order Lactobacillales, in the phylum Bacillota. Cell division in streptococci occurs along a single axis, so as they grow, they tend to form pairs or chains that may appear bent or twisted. This differs from staphylococci, which divide along multiple axes, thereby generating irregular, grape-like clusters of cells. Most streptococci are oxidase-negative and catalase-negative, and many are facultative anaerobes.

Group A streptococcal infections are a number of infections with Streptococcus pyogenes, a group A streptococcus (GAS). S. pyogenes is a species of beta-hemolytic Gram-positive bacteria that is responsible for a wide range of infections that are mostly common and fairly mild. If the bacteria enter the bloodstream an infection can become severe and life-threatening, and is called an invasive GAS (iGAS).

Streptococcus pyogenes is a species of Gram-positive, aerotolerant bacteria in the genus Streptococcus. These bacteria are extracellular, and made up of non-motile and non-sporing cocci that tend to link in chains. They are clinically important for humans, as they are an infrequent, but usually pathogenic, part of the skin microbiota that can cause Group A streptococcal infection. S. pyogenes is the predominant species harboring the Lancefield group A antigen, and is often called group A Streptococcus (GAS). However, both Streptococcus dysgalactiae and the Streptococcus anginosus group can possess group A antigen as well. Group A streptococci, when grown on blood agar, typically produce small (2–3 mm) zones of beta-hemolysis, a complete destruction of red blood cells. The name group A (beta-hemolytic) Streptococcus is thus also used.
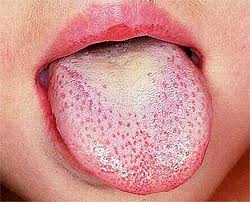
Scarlet fever, also known as scarlatina, is an infectious disease caused by Streptococcus pyogenes, a Group A streptococcus (GAS). It most commonly affects children between five and 15 years of age. The signs and symptoms include a sore throat, fever, headache, swollen lymph nodes, and a characteristic rash. The face is flushed and the rash is red and blanching. It typically feels like sandpaper and the tongue may be red and bumpy. The rash occurs as a result of capillary damage by exotoxins produced by S.pyogenes. On darker-pigmented skin the rash may be hard to discern.

Streptococcal pharyngitis, also known as streptococcal sore throat, is pharyngitis caused by Streptococcus pyogenes, a gram-positive, group A streptococcus. Common symptoms include fever, sore throat, red tonsils, and enlarged lymph nodes in the front of the neck. A headache and nausea or vomiting may also occur. Some develop a sandpaper-like rash which is known as scarlet fever. Symptoms typically begin one to three days after exposure and last seven to ten days.
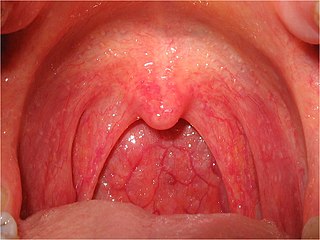
Pharyngitis is inflammation of the back of the throat, known as the pharynx. It typically results in a sore throat and fever. Other symptoms may include a runny nose, cough, headache, difficulty swallowing, swollen lymph nodes, and a hoarse voice. Symptoms usually last 3–5 days, but can be longer depending on cause. Complications can include sinusitis and acute otitis media. Pharyngitis is a type of upper respiratory tract infection.

Sore throat, also known as throat pain, is pain or irritation of the throat. Usually, causes of sore throat include:

Infective endocarditis is an infection of the inner surface of the heart, usually the valves. Signs and symptoms may include fever, small areas of bleeding into the skin, heart murmur, feeling tired, and low red blood cell count. Complications may include backward blood flow in the heart, heart failure – the heart struggling to pump a sufficient amount of blood to meet the body's needs, abnormal electrical conduction in the heart, stroke, and kidney failure.

Tonsillitis is inflammation of the tonsils in the upper part of the throat. It can be acute or chronic. Acute tonsillitis typically has a rapid onset. Symptoms may include sore throat, fever, enlargement of the tonsils, trouble swallowing, and enlarged lymph nodes around the neck. Complications include peritonsillar abscess (Quinsy).
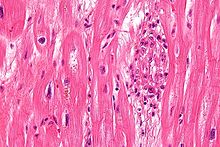
Sydenham's chorea, also known as rheumatic chorea, is a disorder characterized by rapid, uncoordinated jerking movements primarily affecting the face, hands and feet. Sydenham's chorea is an autoimmune disease that results from childhood infection with Group A beta-haemolytic Streptococcus. It is reported to occur in 20–30% of people with acute rheumatic fever and is one of the major criteria for it, although it sometimes occurs in isolation. The disease occurs typically a few weeks, but up to 6 months, after the acute infection, which may have been a simple sore throat (pharyngitis).

Erythema marginatum is an acquired skin condition which primarily affects the arms, trunk, and legs. It is a type of erythema characterised by bright pink or red circular lesions which have sharply-defined borders and faint central clearing. The lesions typically range from 3 to 10 cm in size, and are distributed symmetrically over the torso and inner surfaces of the limbs and extensor surfaces. The lesions last between one and four weeks but have been known to be present on patients for as long as several months.
A complication in medicine, or medical complication, is an unfavorable result of a disease, health condition, or treatment. Complications may adversely affect the prognosis, or outcome, of a disease. Complications generally involve a worsening in the severity of the disease or the development of new signs, symptoms, or pathological changes that may become widespread throughout the body and affect other organ systems. Thus, complications may lead to the development of new diseases resulting from previously existing diseases. Complications may also arise as a result of various treatments.

Valvular heart disease is any cardiovascular disease process involving one or more of the four valves of the heart. These conditions occur largely as a consequence of aging, but may also be the result of congenital (inborn) abnormalities or specific disease or physiologic processes including rheumatic heart disease and pregnancy.
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) is a controversial hypothetical diagnosis for a subset of children with rapid onset of obsessive-compulsive disorder (OCD) or tic disorders. Symptoms are proposed to be caused by group A streptococcal (GAS), and more specifically, group A beta-hemolytic streptococcal (GABHS) infections. OCD and tic disorders are hypothesized to arise in a subset of children as a result of a post-streptococcal autoimmune process. The proposed link between infection and these disorders is that an autoimmune reaction to infection produces antibodies that interfere with basal ganglia function, causing symptom exacerbations, and this autoimmune response results in a broad range of neuropsychiatric symptoms.
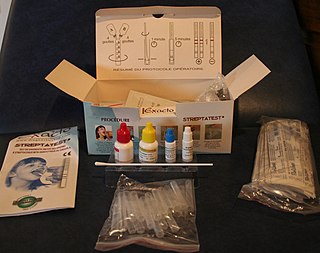
The rapid strep test (RST) is a rapid antigen detection test (RADT) that is widely used in clinics to assist in the diagnosis of bacterial pharyngitis caused by group A streptococci (GAS), sometimes termed strep throat. There are currently several types of rapid strep test in use, each employing a distinct technology. However, they all work by detecting the presence of GAS in the throat of a person by responding to GAS-specific antigens on a throat swab.
M protein is a virulence factor that can be produced by certain species of Streptococcus.
Perianal cellulitis, also known as perianitis or perianal streptococcal dermatitis, is a bacterial infection affecting the lower layers of the skin (cellulitis) around the anus. It presents as bright redness in the skin and can be accompanied by pain, difficulty defecating, itching, and bleeding. This disease is considered a complicated skin and soft tissue infection (cSSTI) because of the involvement of the deeper soft tissues.
Bacteriophage T12 is a bacteriophage that infects Streptococcus pyogenes bacteria. It is a proposed species of the family Siphoviridae in the order Caudovirales also known as tailed viruses. It converts a harmless strain of bacteria into a virulent strain. It carries the speA gene which codes for erythrogenic toxin A. speA is also known as streptococcal pyogenic exotoxin A, scarlet fever toxin A, or even scarlatinal toxin. Note that the name of the gene "speA" is italicized; the name of the toxin "speA" is not italicized. Erythrogenic toxin A converts a harmless, non-virulent strain of Streptococcus pyogenes to a virulent strain through lysogeny, a life cycle which is characterized by the ability of the genome to become a part of the host cell and be stably maintained there for generations. Phages with a lysogenic life cycle are also called temperate phages. Bacteriophage T12, proposed member of family Siphoviridae including related speA-carrying bacteriophages, is also a prototypic phage for all the speA-carrying phages of Streptococcus pyogenes, meaning that its genome is the prototype for the genomes of all such phages of S. pyogenes. It is the main suspect as the cause of scarlet fever, an infectious disease that affects small children.
Anti-Deoxyribonuclease B titres are a quantitative measure of the presence of serologic antibodies obtained from patients suspected of having a recent group A (Beta-hemolytic) streptococcus bacteria infection, from Streptococcus pyogenes.
Shiranee Sriskandan is a British academic who is Professor of Infectious Diseases at Imperial College London and Honorary Consultant at Hammersmith Hospital. Her research considers how Gram-positive bacteria cause disease, with a particular focus on the bacteria Streptococcus pyogenes.