
Alfentanil is a potent but short-acting synthetic opioid analgesic drug, used for anaesthesia in surgery. It is an analogue of fentanyl with around one-fourth to one-tenth the potency, one-third the duration of action, and an onset of action four times faster than that of fentanyl. Alfentanil has a pKa of approximately 6.5, which leads to a very high proportion of the drug being uncharged at physiologic pH, a characteristic responsible for its rapid onset. It is an agonist at mu opioid receptors.

3-Methylfentanyl is an opioid analgesic that is an analog of fentanyl. 3-Methylfentanyl is one of the most potent opioids, estimated to be between 400 and 6000 times stronger than morphine, depending on which isomer is used.

Betahydroxythiofentanyl (β-hydroxythiofentanyl) is an opioid analgesic that is an analogue of fentanyl. Beginning as early as 2008, clandestine labs in China began to manufacture synthetic opioids on an industrial scale. Initially, these opioids were distributed in Eastern European markets such as Ukraine and Estonia. Beginning in 2015, fentanyl had replaced heroin as the opioid of choice due to its cheap cost of production and astronomical potency. Utilizing lax regulation in the ports of western Mexico, Mexican and Chinese criminal organizations began to traffic the drug en masse along the US/Mexico border.

3-Methyl-thiofentanyl is an opioid analgesic and analogue of fentanyl.

β-Hydroxyfentanyl (Fentanol) is an opioid analgesic that is an analogue of fentanyl.

Brifentanil (A-3331) is an opioid analgesic that is an analogue of fentanyl and was developed in the early 1990s.

Lofentanil is one of the most potent opioid analgesics known and is an analogue of fentanyl, which was developed in 1960. It is most similar to the highly potent opioid carfentanil (4-carbomethoxyfentanyl), only slightly more potent. Lofentanil can be described as 3-methylcarfentanil, or 3-methyl-4-carbomethoxyfentanyl. While 3-methylfentanyl is considerably more potent than fentanyl itself, lofentanil is only slightly stronger than carfentanil. This suggests that substitution at both the 3 and 4 positions of the piperidine ring introduces steric hindrance which prevents μ-opioid affinity from increasing much further. As with other 3-substituted fentanyl derivatives such as ohmefentanyl, the stereoisomerism of lofentanil is very important, with some stereoisomers being much more potent than others.

3-Allylfentanyl is an opioid analgesic that is an analogue of fentanyl.
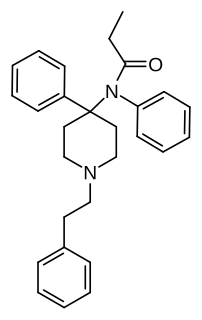
4-Phenylfentanyl is an opioid analgesic that is a derivative of fentanyl. It was developed during the course of research that ultimately resulted in super-potent opioid derivatives such as carfentanil, though it is a substantially less potent analogue. 4-Phenylfentanyl is around eight times the potency of fentanyl in analgesic tests on animals, but more complex 4-heteroaryl derivatives such as substituted thiophenes and thiazoles are more potent still, as they are closer bioisosteres to the 4-carbomethoxy group of carfentanil.

Butyrfentanyl or butyrylfentanyl is a potent short-acting synthetic opioid analgesic drug. It is an analog of fentanyl with around one quarter of its potency. One of the first mentions of this drug can be found in document written by The College on Problem of Drug Dependence, where it is mentioned as N-butyramide fentanyl analog. This document also states that the article describing its clinical effects was published in 1987. It is an agonist for the μ-opioid receptors.

3-Methylbutyrfentanyl (3-MBF) is an opioid analgesic that is an analog of butyrfentanyl.
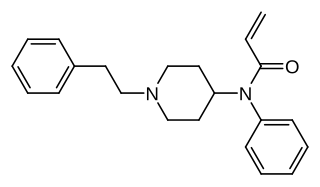
Acrylfentanyl (also known as acryloylfentanyl or Egyptenyl) is a highly potent opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. In animal studies the IC50 or half maximal inhibitory concentration for acrylfentanyl to displace naloxone is 1.4 nM, being slightly more potent than fentanyl itself (1.6 nM) as well as having a longer duration of action.

Methoxyacetylfentanyl, commonly known as MAF is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug.

4-Fluoroisobutyrylfentanyl (also known as 4-FIBF and p-FIBF) is an opioid analgesic that is an analog of butyrfentanyl and structural isomer of 4-Fluorobutyrfentanyl and has been sold online as a designer drug. It is closely related to 4-fluorofentanyl, which has an EC50 value of 4.2 nM for the human μ-opioid receptor. 4-fluoroisobutyrylfentanyl is a highly selective μ-opioid receptor agonist whose analgesic potency is almost ten times of that reported for morphine.

Valerylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. It has been seldom reported on illicit markets and there is little information about it, though it is believed to be less potent than butyrfentanyl but more potent than benzylfentanyl. In one study, it fully substituted for oxycodone and produced antinociception and oxycodone-like discriminative stimulus effects comparable in potency to morphine in mice, but failed to stimulate locomotor activity in mice at doses up to 100 mg/kg.

Isobutyrylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. It is believed to be around the same potency as butyrfentanyl but has been less widely distributed on illicit markets, though it was one of the earliest of the "new wave" of fentanyl derivatives to appear, and was reported in Europe for the first time in December 2012.
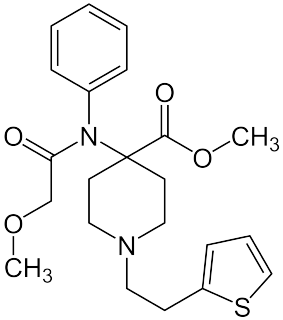
Thiafentanil is a highly potent opioid analgesic that is an analog of fentanyl, and was invented in 1986. Its analgesic potency is slightly less than that of carfentanil, though with a faster onset of effects, shorter duration of action and a slightly lesser tendency to produce respiratory depression. It is used in veterinary medicine to anesthetise animals such as impala, usually in combination with other anesthetics such as ketamine, xylazine or medetomidine to reduce the prevalence of side effects such as muscle rigidity.

Tetramethylcyclopropylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug.

4-Methylphenethylacetylfentanyl is an opioid analgesic that is an analog of fentanyl and has been sold as a designer drug.

Isofentanyl (3-methyl-benzylfentanyl) is an opioid analgesic that is an analog of fentanyl first invented in 1973, and which has been sold as a designer drug.





















