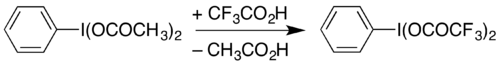| |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Phenyl-λ3-iodanediyl bis(trifluoroacetate) | |
| Other names Phenyliodine bis(trifluoroacetate); PIFA | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.018.462 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C10H5F6IO4 | |
| Molar mass | 430.041 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
(Bis(trifluoroacetoxy)iodo)benzene, C
6H
5I(OCOCF
3)
2, is a hypervalent iodine compound used as a reagent in organic chemistry. It can be used to carry out the Hofmann rearrangement under acidic conditions. [1]