
Cis–trans isomerism, also known as geometric isomerism, describes certain arrangements of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing (transverse) sides. Cis–trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.
Polyketones are a family of high-performance thermoplastic polymers. The polar ketone groups in the polymer backbone of these materials gives rise to a strong attraction between polymer chains, which increases the material's melting point (255 °C for copolymer, 220 °C for terpolymer. Trade names include Poketone, Carilon, Karilon, Akrotek, and Schulaketon. Such materials also tend to resist solvents and have good mechanical properties. Unlike many other engineering plastics, aliphatic polyketones such as Shell Chemicals' Carilon are relatively easy to synthesize and can be derived from inexpensive monomers. Carilon is made with a palladium catalyst from ethylene and carbon monoxide. A small fraction of the ethylene is generally replaced with propylene to reduce the melting point somewhat. Shell Chemical commercially launched Carilon thermoplastic polymer in the U.S. in 1996, but discontinued it in 2000. Hyosung announced that they would launch production in 2015.

Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species along with 1,2-diselenolene derivatives, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is the name for a pair of organochlorine compounds with the molecular formula C2H2Cl2. They are both colorless liquids with a sweet odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have some applications as a degreasing solvent. In contrast to most cis-trans compounds, the Z isomer (cis) is more stable than the E isomer (trans) by 0.4 kcal/mol.
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.

1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (Ph2PCH2)2 (Ph = phenyl). It is a commonly used bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents.

In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.

1,1′-Bis(diphenylphosphino)ferrocene, commonly abbreviated dppf, is an organophosphorus compound commonly used as a ligand in homogeneous catalysis. It contains a ferrocene moiety in its backbone, and is related to other bridged diphosphines such as 1,2-bis(diphenylphosphino)ethane (dppe).
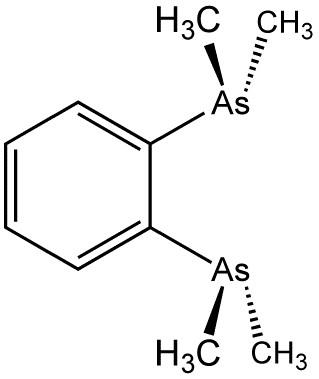
1,2-Bis(dimethylarsino)benzene (diars) is the organoarsenic compound with the formula C6H4(As(CH3)2)2. The molecule consists of two dimethylarsino groups attached to adjacent carbon centers of a benzene ring. It is a chelating ligand in coordination chemistry. This colourless oil is commonly abbreviated "diars."

Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts.

1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle is 73°.
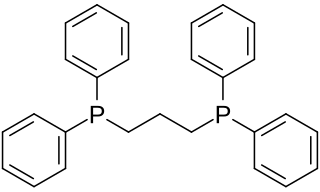
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula Ph2P(CH2)3PPh2. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is classified as a diphosphine ligand in coordination chemistry and homogeneous catalysis.

1,2-Bis(dimethylphosphino)ethane (dmpe) is a diphosphine ligand in coordination chemistry. It is a colorless, air-sensitive liquid that is soluble in organic solvents. With the formula (CH2PMe2)2, dmpe is used as a compact strongly basic spectator ligand (Me = methyl), Representative complexes include V(dmpe)2(BH4)2, Mn(dmpe)2(AlH4)2, Tc(dmpe)2(CO)2Cl, and Ni(dmpe)Cl2.

Nickel(II) bis(acetylacetonate) is a coordination complex with the formula [Ni(acac)2]3, where acac is the anion C5H7O2− derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2.

Dichloro[1,3-bis(diphenylphosphino)propane]nickel a coordination complex with the formula NiCl2(dppp); where dppp is the diphosphine 1,3-bis(diphenylphosphino)propane. It is used as a catalyst in organic synthesis. The compound is a bright orange-red crystalline powder.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

1,5-Diaza-3,7-diphosphacyclooctanes are organophosphorus compounds with the formula [R'NCH2P(R)CH2]2, often abbreviated PR2NR'2. They are air-sensitive white solids that are soluble in organic solvents. The ligands exist as meso and d,l-diastereomers, but only the meso forms function as bidentate ligands.
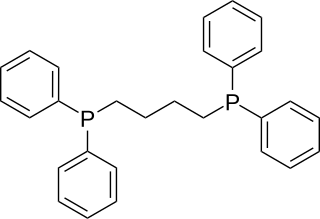
1,4-Bis(diphenylphosphino)butane (dppb) is an organophosphorus compound with the formula (Ph2PCH2CH2)2. It is less commonly used in coordination chemistry than other diphosphine ligands such as dppe. It is a white solid that is soluble in organic solvents.

Dichloro[1,2-bis(diphenylphosphino)ethane]nickel is a coordination complex with the formula NiCl2(dppe); where dppe is the diphosphine 1,2-bis(diphenylphosphino)ethane. It is used as a reagent and as a catalyst. The compound is a bright orange-red diamagnetic solid. The complex adopts a square planar geometry.
1,2-Bis(diphenylphosphino)benzene (dppbz) is an organophosphorus compound with the formula C6H4(PPh2)2 (Ph = C6H5). Classified as a diphosphine ligand, it is a common bidentate ligand in coordination chemistry. It is a white, air-stable solid. As a chelating ligand, dppbz is very similar to 1,2-bis(diphenylphosphino)ethylene.

















