Microfluidics refers to the behavior, precise control, and manipulation of fluids that are geometrically constrained to a small scale at which surface forces dominate volumetric forces. It is a multidisciplinary field that involves engineering, physics, chemistry, biochemistry, nanotechnology, and biotechnology. It has practical applications in the design of systems that process low volumes of fluids to achieve multiplexing, automation, and high-throughput screening. Microfluidics emerged in the beginning of the 1980s and is used in the development of inkjet printheads, DNA chips, lab-on-a-chip technology, micro-propulsion, and micro-thermal technologies.
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2.

Digital microfluidics (DMF) is a platform for lab-on-a-chip systems that is based upon the manipulation of microdroplets. Droplets are dispensed, moved, stored, mixed, reacted, or analyzed on a platform with a set of insulated electrodes. Digital microfluidics can be used together with analytical analysis procedures such as mass spectrometry, colorimetry, electrochemical, and electrochemiluminescense.
Chemical biology is a scientific discipline between the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and manipulation of biological systems. In contrast to biochemistry, which involves the study of the chemistry of biomolecules and regulation of biochemical pathways within and between cells, chemical biology deals with chemistry applied to biology.
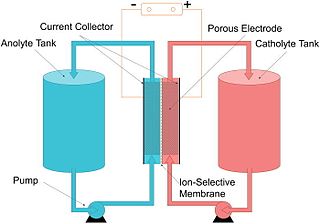
A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion transfer inside the cell occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts. The energy capacity is a function of the electrolyte volume and the power is a function of the surface area of the electrodes.

Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.

Christopher Abell was a British biological chemist. He was Professor of Biological Chemistry at the Department of Chemistry of the University of Cambridge and Todd-Hamied Fellow of Christ's College, Cambridge. On his 2016 election to the Royal Society, the society described his research as having "changed the face of drug discovery."
In chemistry, a (redox) non-innocent ligand is a ligand in a metal complex where the oxidation state is not clear. Typically, complexes containing non-innocent ligands are redox active at mild potentials. The concept assumes that redox reactions in metal complexes are either metal or ligand localized, which is a simplification, albeit a useful one.
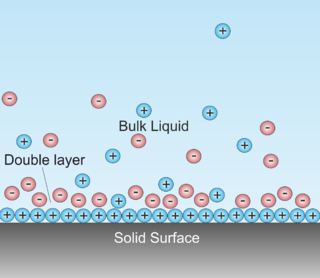
A double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".
The electrochemical reduction of carbon dioxide, also known as electrolysis of carbon dioxide, is the conversion of carbon dioxide to more reduced chemical species using electrical energy. It is one possible step in the broad scheme of carbon capture and utilization, nevertheless it is deemed to be one of the most promising approaches.
Scanning electrochemical microscopy (SECM) is a technique within the broader class of scanning probe microscopy (SPM) that is used to measure the local electrochemical behavior of liquid/solid, liquid/gas and liquid/liquid interfaces. Initial characterization of the technique was credited to University of Texas electrochemist, Allen J. Bard, in 1989. Since then, the theoretical underpinnings have matured to allow widespread use of the technique in chemistry, biology and materials science. Spatially resolved electrochemical signals can be acquired by measuring the current at an ultramicroelectrode (UME) tip as a function of precise tip position over a substrate region of interest. Interpretation of the SECM signal is based on the concept of diffusion-limited current. Two-dimensional raster scan information can be compiled to generate images of surface reactivity and chemical kinetics.
The organic electrochemical transistor (OECT) is an organic electronic device which functions like a transistor. The current flowing through the device is controlled by the exchange of ions between an electrolyte and the OECT channel composed of an organic conductor or semiconductor. The exchange of ions is driven by a voltage applied to the gate electrode which is in ionic contact with the channel through the electrolyte. The migration of ions between the channel and the electrolyte is accompanied by electrochemical redox reactions occurring in the channel material. The electrochemical redox of the channel along with ion migration changes the conductivity of the channel in a process called electrochemical doping. OECTs are being explored for applications in biosensors, bioelectronics and large-area, low-cost electronics. OECTs can also be used as multi-bit memory devices that mimic the synaptic functionalities of the brain. For this reason, OECTs can be also being investigated as elements in neuromorphic computing applications.
In electrochemistry, protein film voltammetry is a technique for examining the behavior of proteins immobilized on an electrode. The technique is applicable to proteins and enzymes that engage in electron transfer reactions and it is part of the methods available to study enzyme kinetics.
Klavs Flemming Jensen is a chemical engineer who is currently the Warren K. Lewis Professor at the Massachusetts Institute of Technology (MIT).
Droplet-based microfluidics manipulate discrete volumes of fluids in immiscible phases with low Reynolds number and laminar flow regimes. Interest in droplet-based microfluidics systems has been growing substantially in past decades. Microdroplets offer the feasibility of handling miniature volumes of fluids conveniently, provide better mixing, encapsulation, sorting, sensing and are suitable for high throughput experiments. Two immiscible phases used for the droplet based systems are referred to as the continuous phase and dispersed phase.
Paper-based microfluidics are microfluidic devices that consist of a series of hydrophilic cellulose or nitrocellulose fibers that transport fluid from an inlet through the porous medium to a desired outlet or region of the device, by means of capillary action. This technology builds on the conventional lateral flow test which is capable of detecting many infectious agents and chemical contaminants. The main advantage of this is that it is largely a passively controlled device unlike more complex microfluidic devices. Development of paper-based microfluidic devices began in the early 21st century to meet a need for inexpensive and portable medical diagnostic systems.

A semi-solid flow battery is a type of flow battery using solid battery active materials or involving solid species in the energy carrying fluid. A research team in MIT proposed this concept using lithium-ion battery materials. In such a system, both positive (cathode) and negative electrode (anode) consist of active material particles with carbon black suspended in liquid electrolyte. Active material suspensions are stored in two energy storage tanks. The suspensions are pumped into the electrochemical reaction cell when charging and discharging. This design takes advantage of both the designing flexibility of flow batteries and the high energy density active materials of lithium-ion batteries.
Calcium (ion) batteries are energy storage and delivery technologies (i.e., electro–chemical energy storage) that employ calcium ions (cations), Ca2+, as the active charge carrier in the electrolytes as well as in the electrodes (anode and cathode). Calcium (ion) batteries remain an active area of research, with studies and work persisting in the discovery and development of electrodes and electrolytes that enable stable, long-term battery operation.
Amol Arvindrao Kulkarni is an Indian research scientist at National Chemical Laboratory, Pune. He earned his PhD from the Institute of Chemical Technology, Mumbai in chemical engineering. His research expertise includes design and development of microreactors.
Susan A. Odom was a Professor of Chemistry at the University of Kentucky who developed redox active organic compounds for energy storage applications.






