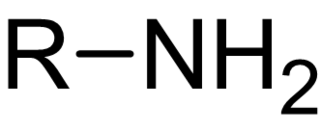
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two organyl groups. They have the general formula R−O−R′, where R and R′ represent organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
Amination is the process by which an amine group is introduced into an organic molecule. This type of reaction is important because organonitrogen compounds are pervasive.

Azo compounds are organic compounds bearing the functional group diazenyl.

Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.

In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure [NR4]+, where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.
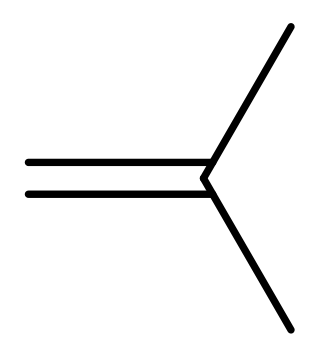
Isobutylene is a hydrocarbon with the chemical formula (CH3)2C=CH2. It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". In addition to pyrrolidine itself, many substituted pyrrolidines are known.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.

Diethylenetriamine (abbreviated Dien or DETA) and also known as 2,2’-Iminodi(ethylamine)) is an organic compound with the formula HN(CH2CH2NH2)2. This colourless hygroscopic liquid is soluble in water and polar organic solvents, but not simple hydrocarbons. Diethylenetriamine is structural analogue of diethylene glycol. Its chemical properties resemble those for ethylene diamine, and it has similar uses. It is a weak base and its aqueous solution is alkaline. DETA is a byproduct of the production of ethylenediamine from ethylene dichloride.

Proton nuclear magnetic resonance is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen (H) is used, practically all the hydrogen consists of the isotope 1H.
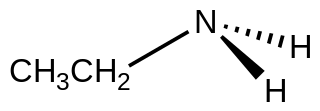
Ethylamine, also known as ethanamine, is an organic compound with the formula CH3CH2NH2. This colourless gas has a strong ammonia-like odor. It condenses just below room temperature to a liquid miscible with virtually all solvents. It is a nucleophilic base, as is typical for amines. Ethylamine is widely used in chemical industry and organic synthesis.

Propylamine, also known as n-propylamine, is an amine with the chemical formula CH3(CH2)2NH2. It is a colorless volatile liquid.

n-Butylamine is an organic compound (specifically, an amine) with the formula CH3(CH2)3NH2. This colourless liquid is one of the four isomeric amines of butane, the others being sec-butylamine, tert-butylamine, and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Its vapours are heavier than air and it produces toxic oxides of nitrogen during combustion.

Isopropylamine is an organic compound, an amine. It is a hygroscopic colorless liquid with ammonia-like odor. It is miscible with water and flammable. It is a valuable intermediate in chemical industry.

tert-Butylamine (also erbumine and other names) is an organic chemical compound with the formula (CH3)3CNH2. It is a colorless liquid with a typical amine-like odor. tert-Butylamine is one of the four isomeric amines of butane, the others being n-butylamine, sec-butylamine and isobutylamine.

2-Ethylhexanoic acid is the organic compound with the formula CH3(CH2)3CH(C2H5)CO2H. It is a carboxylic acid that is widely used to prepare lipophilic metal derivatives that are soluble in nonpolar organic solvents. 2-Ethylhexanoic acid is a colorless viscous oil. It is supplied as a racemic mixture.
In chemistry, primene amines are mixtures of long-chain branched primary amines. One member of this class of amine is tert-octylamine, H2NC(CH3)2(CH2)4CH3. These compounds have a faint ammonia-like odor. The compounds are colorless, although typical commercial samples are yellowish owing to the presence of impurities. Primene amines are used as used in solvent extractions.



















