Amination is the process by which an amine group is introduced into an organic molecule. This type of reaction is important because organonitrogen compounds are pervasive.
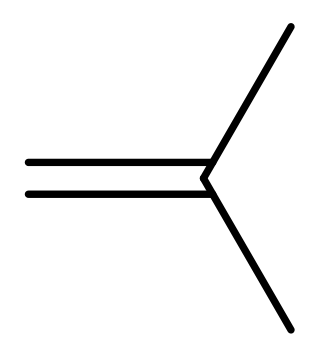
Isobutylene is a hydrocarbon with the chemical formula (CH3)2C=CH2. It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
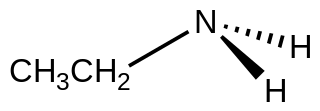
Ethylamine, also known as ethanamine, is an organic compound with the formula CH3CH2NH2. This colourless gas has a strong ammonia-like odor. It condenses just below room temperature to a liquid miscible with virtually all solvents. It is a nucleophilic base, as is typical for amines. Ethylamine is widely used in chemical industry and organic synthesis. It is a DEA list I chemical by 21 CFR § 1310.02.

Ammonia borane, also called borazane, is the chemical compound with the formula H3NBH3. The colourless or white solid is the simplest molecular boron-nitrogen-hydride compound. It has attracted attention as a source for hydrogen fuel, but is otherwise primarily of academic interest.
The carbylamine reaction is the synthesis of an isocyanide by the reaction of a primary amine, chloroform, and base. The conversion involves the intermediacy of dichlorocarbene.

1-Phenyl-2-nitropropene, or simply phenyl-2-nitropropene, or P2NP, as it is commonly referred to, is a chemical compound from the aromatic group of compounds, with the formula C9H9NO2. It is a light-yellow crystalline solid with a distinct smell. Phenyl-2-nitropropene is used in the pharmaceutical industry to manufacture the drug Adderall, an amphetamine mixture used to treat ADHD and narcolepsy. P2NP and other similar nitrostyrenes are also employed in the clandestine manufacture of drugs of the amphetamine class, and are listed as drug precursors in many countries.

n-Butylamine is an organic compound (specifically, an amine) with the formula CH3(CH2)3NH2. This colourless liquid is one of the four isomeric amines of butane, the others being sec-butylamine, tert-butylamine, and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Its vapours are heavier than air and it produces toxic oxides of nitrogen during combustion.
Butylamines are several related chemical compounds:

tert-Butylamine (also erbumine and other names) is an organic chemical compound with the formula (CH3)3CNH2. It is a colorless liquid with a typical amine-like odor. tert-Butylamine is one of the four isomeric amines of butane, the others being n-butylamine, sec-butylamine and isobutylamine.
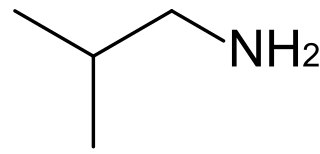
Isobutylamine is an organic chemical compound (specifically, an amine) with the formula (CH3)2CHCH2NH2, and occurs as a colorless liquid. Isobutylamine is one of the four isomeric amines of butane, the others being n-butylamine, sec-butylamine and tert-butylamine. It is the decarboxylated form of the amino acid valine, and the product of the metabolism thereof by the enzyme valine decarboxylase.
The molecular formula C4H11N may refer to:
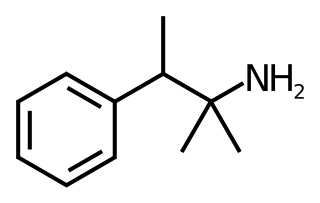
Pentorex, also known as phenpentermine or α,β-dimethylamphetamine and sold under the brand name Modatrop, is a stimulant drug of the amphetamine family related to phentermine (α-methylamphetamine) which is used as an anorectic to assist with weight loss. It also acts as a diuretic. Pentorex was developed by Nordmark in the 1960s.
Triisopropylamine is an organic chemical compound consisting of three isopropyl groups bound to a central nitrogen atom. As a hindered tertiary amine, it can be used as a non-nucleophilic base and as a stabilizer for polymers; however, its applications are limited by its relatively high cost and difficult synthesis.
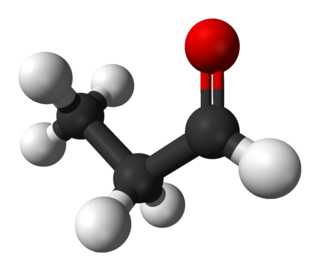
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a pungent and fruity odour. It is produced on a large scale industrially.
The Ritter reaction is a chemical reaction that transforms a nitrile into an N-alkyl amide using various electrophilic alkylating reagents. The original reaction formed the alkylating agent using an alkene in the presence of a strong acid.

In organosulfur chemistry, sulfenamides are a class of organosulfur compounds characterized by the general formula R−S−N(−R)2, where the R groups are hydrogen, alkyl, or aryl. Sulfenamides have been used extensively in the vulcanization of rubber using sulfur. They are related to the oxidized compounds known as sulfinamides and sulfonamides.
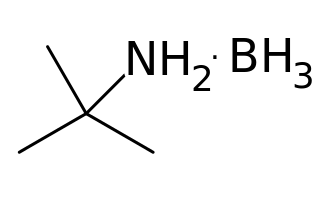
Borane tert-butylamine is an amine borane complex derived from tert-butylamine and borane. It is a colorless solid.

Aminomethyl propanol (AMP) is an organic compound with the formula H2NC(CH3)2CH2OH. It is colorless liquid that is classified as an alkanolamine. It is a useful buffer and a precursor to numerous other organic compounds.

Bunitrolol is a beta-adrenergic antagonist.
Methylpropylamine may refer to:




















