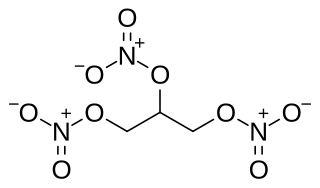
Nitroglycerin (NG), also known as trinitroglycerin (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless, oily, explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid ester. Chemically, the substance is an organic nitrate compound rather than a nitro compound, but the traditional name is retained. Discovered in 1847 by Ascanio Sobrero, nitroglycerin has been used as an active ingredient in the manufacture of explosives, namely dynamite, and as such it is employed in the construction, demolition, and mining industries. It is combined with nitrocellulose to form double-based smokeless powder, which has been used as a propellant in artillery and firearms since the 1880s.

Trinitrotoluene, more commonly known as TNT, more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.

In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.
The regulatory T cells (Tregs or Treg cells), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. Treg cells are immunosuppressive and generally suppress or downregulate induction and proliferation of effector T cells. Treg cells express the biomarkers CD4, FOXP3, and CD25 and are thought to be derived from the same lineage as naïve CD4+ cells. Because effector T cells also express CD4 and CD25, Treg cells are very difficult to effectively discern from effector CD4+, making them difficult to study. Research has found that the cytokine transforming growth factor beta (TGF-β) is essential for Treg cells to differentiate from naïve CD4+ cells and is important in maintaining Treg cell homeostasis.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.

Transforming growth factor beta (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms and many other signaling proteins. TGFB proteins are produced by all white blood cell lineages.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.
Immune tolerance, or immunological tolerance, or immunotolerance, is a state of unresponsiveness of the immune system to substances or tissues that would otherwise have the capacity to elicit an immune response in a given organism. It is induced by prior exposure to that specific antigen and contrasts with conventional immune-mediated elimination of foreign antigens. Tolerance is classified into central tolerance or peripheral tolerance depending on where the state is originally induced—in the thymus and bone marrow (central) or in other tissues and lymph nodes (peripheral). The mechanisms by which these forms of tolerance are established are distinct, but the resulting effect is similar.

A carboxypeptidase is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed.
Nitrophenols are compounds of the formula HOC6H5−x(NO2)x. The conjugate bases are called nitrophenolates. Nitrophenols are more acidic than phenol itself.

Transforming growth factor beta 1 or TGF-β1 is a polypeptide member of the transforming growth factor beta superfamily of cytokines. It is a secreted protein that performs many cellular functions, including the control of cell growth, cell proliferation, cell differentiation, and apoptosis. In humans, TGF-β1 is encoded by the TGFB1 gene.
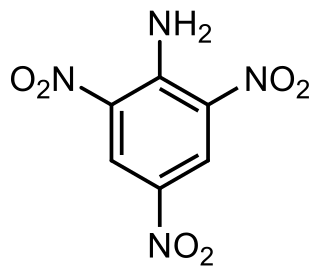
2,4,6-Trinitroaniline, C6H4N4O6, abbreviated as TNA and also known as picramide, a nitrated amine. Materials in this group range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups. The appearance of trinitroaniline varies from yellow to orange to red depending on its purity and concentration.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
Bioconjugation is a chemical strategy to form a stable covalent link between two molecules, at least one of which is a biomolecule.
The Bergmann degradation is a series of chemical reactions designed to remove a single amino acid from the carboxylic acid (C-terminal) end of a peptide. First demonstrated by Max Bergmann in 1934, it is a rarely used method for sequencing peptides. The later developed Edman degradation is an improvement upon the Bergmann degradation, instead cleaving the N-terminal amino acid of peptides to produce a hydantoin containing the desired amino acid.

N-formyl peptide receptor 2 (FPR2) is a G-protein coupled receptor (GPCR) located on the surface of many cell types of various animal species. The human receptor protein is encoded by the FPR2 gene and is activated to regulate cell function by binding any one of a wide variety of ligands including not only certain N-Formylmethionine-containing oligopeptides such as N-Formylmethionine-leucyl-phenylalanine (FMLP) but also the polyunsaturated fatty acid metabolite of arachidonic acid, lipoxin A4 (LXA4). Because of its interaction with lipoxin A4, FPR2 is also commonly named the ALX/FPR2 or just ALX receptor.
T helper 3 cells (Th3) are a subset of T lymphocytes with immunoregulary and immunosuppressive functions, that can be induced by administration of foreign oral antigen. Th3 cells act mainly through the secretion of anti-inflammatory cytokine transforming growth factor beta (TGF-β). Th3 have been described both in mice and human as CD4+FOXP3− regulatory T cells. Th3 cells were first described in research focusing on oral tolerance in the experimental autoimmune encephalitis (EAE) mouse model and later described as CD4+CD25−FOXP3−LAP+ cells, that can be induced in the gut by oral antigen through T cell receptor (TCR) signalling.

Chrysophanol, also known as chrysophanic acid, is a fungal isolate and a natural anthraquinone. It is a C-3 methyl substituted chrysazin of the trihydroxyanthraquinone family.
2,4-Dinitroaniline is a chemical compound with a formula of C6H5N3O4. It is used as an explosive and as a reagent to detect and characterize aldehydes and ketones.
















