The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
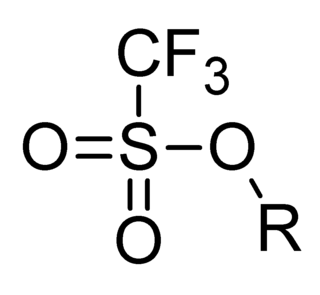
In organic chemistry, triflate, is a functional group with the formula R−OSO2CF3 and structure R−O−S(=O)2−CF3. The triflate group is often represented by −OTf, as opposed to −Tf, which is the triflyl group, R−SO2CF3. For example, n-butyl triflate can be written as CH3CH2CH2CH2OTf.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure R−CH2−C6H5. Benzyl features a benzene ring attached to a methylene group group.
Dimethylformamide is an organic compound with the formula (CH3)2N−C(=O)H. Commonly abbreviated as DMF, this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Dimethylformamide is odorless, but technical-grade or degraded samples often have a fishy smell due to impurity of dimethylamine. Dimethylamine degradation impurities can be removed by sparging samples with an inert gas such as argon or by sonicating the samples under reduced pressure. As its name indicates, it is structurally related to formamide, having two methyl groups in the place of the two hydrogens. DMF is a polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such as SN2 reactions.
The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid or α-substituted β-aryl acrylic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid. The alkali salt acts as a base catalyst, and other bases can be used instead.
A cyanohydrin reaction is an organic chemical reaction in which an aldehyde or ketone reacts with a cyanide anion or a nitrile to form a cyanohydrin. This nucleophilic addition is a reversible reaction but with aliphatic carbonyl compounds equilibrium is in favor of the reaction products. The cyanide source can be potassium cyanide, sodium cyanide or trimethylsilyl cyanide. With aromatic aldehydes such as benzaldehyde, the benzoin condensation is a competing reaction. The reaction is used in carbohydrate chemistry as a chain extension method for example that of D-xylose.
The Vilsmeier–Haack reaction (also called the Vilsmeier reaction) is the chemical reaction of a substituted formamide (1) with phosphorus oxychloride and an electron-rich arene (3) to produce an aryl aldehyde or ketone (5):

(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.

Benzylamine is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water-soluble liquid is a common precursor in organic chemistry and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth.

In organic chemistry, the Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde or formate. The reaction was discovered by Teruaki Mukaiyama in 1973. His choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone, or a different aldehyde without self-condensation of the aldehyde. For this reason the reaction is used extensively in organic synthesis.
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
Lindgren oxidation is a selective method for oxidizing aldehydes to carboxylic acids. The reaction is named after Bengt O. Lindgren.
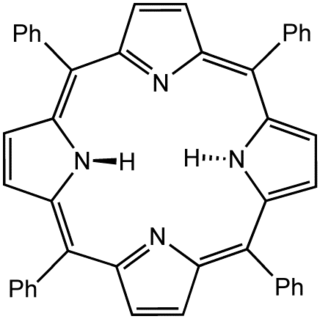
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin B12. The study of naturally occurring porphyrins is complicated by their low symmetry and the presence of polar substituents. Tetraphenylporphyrin is hydrophobic, symmetrically substituted, and easily synthesized. The compound is a dark purple solid that dissolves in nonpolar organic solvents such as chloroform and benzene.

2-Nitrobenzaldehyde is an organic aromatic compound containing a nitro group ortho to formyl. 2-Nitrobenzaldehyde once was produced as an intermediate in the synthesis of the popular dye Indigo.
A tosylhydrazone in organic chemistry is a functional group with the general structure RR'C=N-NH-Ts where Ts is a tosyl group. Organic compounds having this functional group can be accessed by reaction of an aldehyde or ketone with tosylhydrazine.

In organic chemistry, a benzylidene acetal is the functional group with the structural formula C6H5CH(OR)2 (R = alkyl, aryl). Benzylidene acetals are used as protecting groups in glycochemistry. These compounds can also be oxidized to carboxylic acids in order to open important biological molecules, such as glycosaminoglycans, to other routes of synthesis. They arise from the reaction of a 1,2- or 1,3-diols with benzaldehyde. Other aromatic aldehydes are also used.
In organic chemistry, the Hass–Bender oxidation is an organic oxidation reaction that converts benzyl halides into benzaldehydes using the sodium salt of 2-nitropropane as the oxidant. This name reaction is named for Henry B. Hass and Myron L. Bender, who first reported it in 1949.
The Bobbitt reaction is a name reaction in organic chemistry. It is named after the American chemist James M. Bobbitt. The reaction allows the synthesis of 1-, 4-, and N-substituted 1,2,3,4-tetrahydroisoquinolines and also 1-, and 4-substituted isoquinolines.












