
A carcinogen is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruses and bacteria. Most carcinogens act by creating mutations in DNA that disrupt a cell's normal processes for regulating growth, leading to uncontrolled cellular proliferation. This occurs when the cell's DNA repair processes fail to identify DNA damage allowing the defect to be passed down to daughter cells. The damage accumulates over time. This is typically a multi-step process during which the regulatory mechanisms within the cell are gradually dismantled allowing for unchecked cellular division.
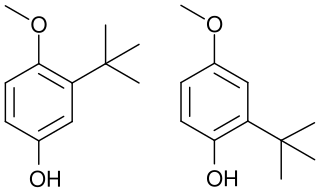
Butylated hydroxyanisole (BHA) is a synthetic, waxy, solid petrochemical. Its antioxidant properties have caused it to be widely used as a preservative in food, food packaging, animal feed, cosmetics, pharmaceuticals, rubber, and petroleum products. BHA has been used in food since around 1947.

Bromoform is an organic compound with the chemical formula CHBr3. It is a colorless liquid at room temperature, with a high refractive index and a very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. It is a brominated organic solvent. Currently its main use is as a laboratory reagent. It is very slightly soluble in water and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone and oils.
The International Agency for Research on Cancer is an intergovernmental agency forming part of the World Health Organization of the United Nations. Its role is to conduct and coordinate research into the causes of cancer. It also collects and publishes surveillance data regarding the occurrence of cancer worldwide.
IARC group 1 Carcinogens are substances, chemical mixtures, and exposure circumstances which have been classified as carcinogenic to humans by the International Agency for Research on Cancer (IARC). This category is used when there is sufficient evidence of carcinogenicity in humans. Exceptionally, an agent may be placed in this category when evidence of carcinogenicity in humans is less than sufficient, but when there is sufficient evidence of carcinogenicity in experimental animals and strong evidence in exposed humans that the agent (mixture) acts through a relevant mechanism of carcinogenicity.
IARC group 2A agents are substances and exposure circumstances that have been classified as probable carcinogens by the International Agency for Research on Cancer (IARC). This designation is applied when there is limited evidence of carcinogenicity in humans, as well as sufficient evidence of carcinogenicity in experimental animals. In some cases, an agent may be classified in this group when there is inadequate evidence of carcinogenicity in humans along with sufficient evidence of carcinogenicity in experimental animals and strong evidence that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this group solely on the basis of limited evidence of carcinogenicity in humans.
IARC group 2B substances, mixtures and exposure circumstances are those that have been classified as "possibly carcinogenic to humans" by the International Agency for Research on Cancer (IARC) as This category is used when there is limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals. It may also be used when there is insufficient evidence of carcinogenicity in humans but sufficient evidence in experimental animals. In some cases, an agent, mixture, or exposure circumstance with inadequate evidence of carcinogenicity in humans but limited evidence in experimental animals, combined with supporting evidence from other relevant data, may be included in this group.

N-Nitrosonornicotine (NNN) is a tobacco-specific nitrosamine produced during the curing and processing of tobacco.

Potassium bromate is a bromate of potassium and takes the form of white crystals or powder. It is a strong oxidizing agent.

Caffeic acid is an organic compound with the formula (HO)2C6H3CH=CHCO2H. It is a polyphenol. It is a yellow solid. Structurally, it is classified as a hydroxycinnamic acid. The molecule consists of both phenolic and acrylic functional groups. It is found in all plants as an intermediate in the biosynthesis of lignin, one of the principal components of biomass and its residues. It is unrelated to caffeine.
Hexachlorobenzene, or perchlorobenzene, is an aryl chloride and a six-substituted chlorobenzene with the molecular formula C6Cl6. It is a fungicide formerly used as a seed treatment, especially on wheat to control the fungal disease bunt. It has been banned globally under the Stockholm Convention on Persistent Organic Pollutants.
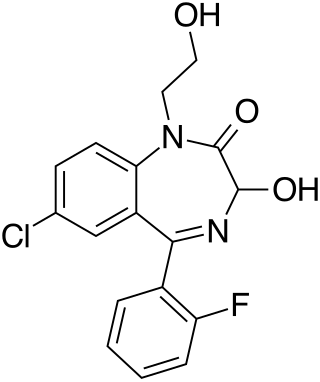
Doxefazepam is a benzodiazepine medication It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. It is used therapeutically as a hypnotic. According to Babbini and colleagues in 1975, this derivative of flurazepam was between 2 and 4 times more potent than the latter while at the same time being half as toxic in laboratory animals.

N-Nitrosodiethylamine (NDEA) is an organic compound with the formula Et2NNO (Et = C2H5). A member of the nitrosamines, it is a light-sensitive, volatile, clear yellow oil that is soluble in water, lipids, and other organic solvents. It has an amine or aromatic odor. It is used as gasoline and lubricant additive, antioxidant, and stabilizer for industry materials. When heated to decomposition, N-nitrosodiethylamine emits toxic fumes of nitrogen oxides. N-Nitrosodiethylamine affects DNA integrity, probably by alkylation, and is used in experimental research to induce liver tumorigenesis. It is carcinogenic and mutagenic. NDEA has also been found to perturb amino acid biosynthesis including arginine, as well as DNA damage repair and mitochondrial genome maintenance in yeast.

2-Acetylaminofluorene is a carcinogenic and mutagenic derivative of fluorene. It is used as a biochemical tool in the study of carcinogenesis. It induces tumors in a number of species in the liver, bladder and kidney. The metabolism of this compound in the body by means of biotransformation reactions is the key to its carcinogenicity. 2-AAF is a substrate for cytochrome P-450 (CYP) enzyme, which is a part of a super family found in almost all organisms. This reaction results in the formation of hydroxyacetylaminofluorene which is a proximal carcinogen and is more potent than the parent molecule. The N-hydroxy metabolite undergoes several enzymatic and non-enzymatic rearrangements. It can be O-acetylated by cytosolic N-acetyltransferase enzyme to yield N-acetyl-N-acetoxyaminofluorene. This intermediate can spontaneously rearrange to form the arylamidonium ion and a carbonium ion which can interact directly with DNA to produce DNA adducts. In addition to esterification by acetylation, the N-hydroxy derivative can be O-sulfated by cytosolic sulfur transferase enzyme giving rise to the N-acetyl-N-sulfoxy product.

PhIP (2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) is one of the most abundant heterocyclic amines (HCAs) in cooked meat. PhIP is formed at high temperatures from the reaction between creatine or creatinine, amino acids, and sugar. PhIP formation increases with the temperature and duration of cooking and also depends on the method of cooking and the variety of meat being cooked. The U.S. Department of Health and Human Services National Toxicology Program has declared PhIP as "reasonably anticipated to be a human carcinogen". International Agency for Research on Cancer (IARC), part of World Health Organization, has classified PhIP as IARC Group 2B carcinogen. There is sufficient evidence in experimental animals, as well as in vitro models, for the carcinogenicity of PhIP.
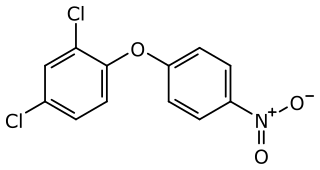
Nitrofen is an herbicide of the diphenyl ether class. Because of concerns about its carcinogenicity, the use of nitrofen has been banned in the European Union and in the United States since 1996. It has been superseded by related protoporphyrinogen oxidase enzyme inhibitors including acifluorfen and fomesafen.

Diepoxybutane is an epoxide which is a colorless liquid at room temperature. It is therefore highly reactive, more than other ethers. An epoxide is a cyclic ether that contains a three atom ring that comes close to an equilateral triangle. The primary structure of an epoxide contains two carbon atoms and a hydrocarbon attached to an oxygen atom. It polymerizes in the presence of catalysts or when heated. It’s hydrophilic, very flammable and easily ignited by heat or sparks.

Nornicotine is an alkaloid found in various plants including Nicotiana, the tobacco plant. It is chemically similar to nicotine, but does not contain a methyl group.
Butyl acrylate is an organic compound with the formula C4H9O2CCH=CH2. A colorless liquid, it is the butyl ester of acrylic acid. It is used commercially on a large scale as a precursor to poly(butyl acrylate). Especially as copolymers, such materials are used in paints, sealants, coatings, adhesives, fuel, textiles, plastics, and caulk.

Indeno(1,2,3-cd)pyrene is a polycyclic aromatic hydrocarbon (PAH), one of 16 PAHs generally measured in studies of environmental exposure and air pollution. Many compounds of this class are formed when burning coal, oil, gas, wood, household waste and tobacco, and can bind to or form small particles in the air. The compounds are known to have toxic, mutagenic and/or carcinogenic properties. Over 100 different PAHs have been identified in environmental samples, including indeno(1,2,3-cd)pyrene (IP). In 1962, the National Cancer Institute reported that indeno(1,2,3-cd)pyrene has a slight tumor activity. This was confirmed in 1973 by the IARC in mice testing.















